The most powerful explosive. Explosive substances: classification, examples, application and storage. Basic properties of explosives
Each new generation tries to outdo the previous generations in what is called the stuffing for hellish machines and other things, in other words - in the search for a powerful explosive. It would seem that the era of explosives in the form of gunpowder is gradually fading away, but the search for new explosives does not stop. The smaller the mass of the explosive and the greater its destructive power, the better it seems to military experts. Robotics dictates an intensification of the search for such an explosive, as well as the use of small missiles and bombs of high destructive power on UAVs.
Naturally, an ideal substance from a military point of view is unlikely to ever be discovered, but recent developments suggest that something close to such a concept can still be obtained. Close to ideality here means stable storage, high destructive power, small volume and easy transportation. We must not forget that the price of such an explosive must also be acceptable, otherwise the creation of weapons based on it can simply devastate the military budget of a particular country.
Developments have been going on around the use of chemical formulas substances such as trinitrotoluene, pentrite, hexogen and a number of others. However, it is extremely rare for “explosive” science to offer fully new products.
That is why the appearance of such a substance as hexantirogexaazaisowurtzitane (the name is tongue-tied) can be considered a real breakthrough in its field. In order not to break the tongue, scientists decided to give this substance a more digestible name - CL-20.
This substance was first obtained about 26 years ago - back in 1986 in the American state of California. Its peculiarity lies in the fact that the energy density in this substance is still maximum in comparison with other substances. High energy density CL-20 and little competition in its production lead to the fact that the cost of such explosives today is simply astronomical. One kilogram of CL-20 costs about $1,300. Naturally, this price does not allow the use of an explosive agent on an industrial scale. However, soon, experts believe, the price of this explosive may drop significantly, since there are options for alternative synthesis of hexantirogexaazaisowurtzitane.
If we compare hexanthirogexaazaisowurtzitane with the most effective explosive used for military purposes today (octogen), then the cost of the latter is about one hundred dollars per kg. However, it is hexanthirogexaazaisowurtzitane that is more effective. The detonation speed of CL-20 is 9660 m/s, which is 560 m/s more than that of HMX. The density of CL-20 is also higher than that of the same HMX, which means that the prospects for hexanthirogexaazaisowurtzitane should also be fine.
One of the possible areas for using the CL-20 today is drones. However, there is a problem here because the CL-20 is very sensitive to mechanical influences. Even ordinary shaking, which may well occur with a UAV in the air, can cause detonation of the substance. To avoid the explosion of the drone itself, experts proposed using the CL-20 in integration with a plastic component that would reduce the level of mechanical impact. But as soon as such experiments were carried out, it turned out that hexanthirogexaazaisowurtzitane (formula C6H6N12O12) greatly loses its “killer” properties.
It turns out that this substance has enormous prospects, but for two and a half decades no one has been able to manage it wisely. But experiments continue today. American Adam Matzger is working on improving CL-20, trying to change the shape of this matter.

Matzger decided to use crystallization from a common solution to obtain molecular crystals of the substance. As a result, they came up with a variant where for every 2 molecules of CL-20 there is 1 molecule of HMX. The detonation speed of this mixture is between the speeds of the two indicated substances separately, but the new substance is much more stable than CL-20 itself and more effective than HMX.
What is the most effective explosive in the world?..
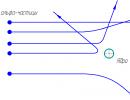
Results of tests of explosives for penetrating ability: on the right - for a 30-gram HMX charge, on the left - for the same CL-20 charge


The search for ever more powerful explosives has continued for centuries. Traditional gunpowder has long since disappeared from the scene, but the emergence of compact robotic means of warfare, including drones, only stimulates new searches. The smaller size and mass of warheads will retain the killing power of their larger predecessors only thanks to the latest achievements of chemists.
The ideal explosive is necessarily a balance between maximum explosive power and maximum stability during storage and transportation. This is also the maximum density of chemical energy, the minimum cost of production and, preferably, environmental safety. Achieving all this is not easy, so for developments in this area they usually take already proven formulas - TNT, hexogen, pentrite, hexanitrostilbene, etc. - and try to improve one of the desired characteristics without compromising the others. Completely new compounds appear extremely rarely.
An interesting exception to this rule may be hexanitrohexaazaisowurtzitane (CL-20), which is poised to join the elite list of popular explosives. First synthesized in California in 1986 (hence the CL in its abbreviated name), it contains chemical energy in the most dense form. So far, it is industrially produced by a few companies at a price of more than $1,300 per kilogram, but with the transition to large-scale synthesis, the cost may fall, according to experts, by 5-10 times.
Today, one of the most effective military explosives is HMX, which is used in plastic charges and costs about $100 per kilogram. However, the CL-20 (look at the illustration on the left) shows noticeably more power: in tests of penetration through steel blocks, it is 40% more effective. This power is provided by a higher detonation speed (9660 m/s versus 9100 m/s) and a higher density of the substance (2.04 g/cm3 versus 1.91).
This incredible power suggests that the CL-20 will be especially useful when used with compact combat systems, such as modern drones. However, it is dangerously sensitive to shock and shock - much like pentrite, the most sensitive compound of all explosives used. It was initially assumed that CL-20 could be used in conjunction with a plastic bonding component (in a ratio of 9:1), although in parallel with the reduction in the risk of detonation, the explosive force was also reduced.
In short, the history of the CL-20, which began in the 1980s, has not yet turned out very well. However, chemists do not stop experimenting with it. One of them was the American professor Adam Matzger, under whose leadership the substance seems to have been improved to an acceptable form. The authors tried to change not its structure, but its shape.
It is worth saying here that if you take a mixture of crystals of two different substances, an individual molecule of each crystal finds itself surrounded by neighbors just like it. The properties of the mixture turn out to be something between the properties of both substances in their pure form. Instead, Matzger and his colleagues tried the method of co-crystallization from a common solution - they were able to obtain molecular crystals containing both substances at the same time: for every two molecules of CL-20 there is one molecule of HMX.
Having studied the properties of this compound, scientists found that its detonation speed is 9480 m/s - that is, approximately halfway between the speeds for pure CL-20 and octogen. But the stability is almost as high as that of pure HMX (according to the authors, due to the formation of additional hydrogen bonds between the two types of molecules, which stabilize the sensitive CL-20 molecule). In addition, the crystal density is approximately 20% higher than HMX, making it even more effective. In other words, such a crystal turns out to be a significant improvement in comparison with HMX and a very promising candidate for the role of a new “best explosive in the world.”
EXPLOSIVES- these are substances or their mixtures that, under the influence of external influences (heating, impact, friction, explosion of another substance), can very quickly decompose with the release of gases and a large amount of heat.
Explosive mixtures existed long before man appeared on Earth. The small (1–2 cm in length) orange-blue bombardier beetle Branchynus explodans defends itself from attacks in a very ingenious way. A concentrated solution of hydrogen peroxide accumulates in a small sac in his body. At the right moment, this solution is quickly mixed with the enzyme catalase. The reaction that occurs was observed by anyone who treated a cut finger with a pharmaceutical 3% peroxide solution: the solution literally boils, releasing oxygen bubbles. At the same time, the mixture is heated (the thermal effect of the reaction 2H 2 O 2 ® 2H 2 O + O 2 is 190 kJ/mol). In the beetle, at the same time, another reaction occurs, catalyzed by the enzyme peroxidase: the oxidation of hydroquinone with hydrogen peroxide to benzoquinone (the thermal effect of this reaction is more than 200 kJ/mol). The heat generated is enough to heat the solution to 100° C and even partially evaporate it. The beetle's reaction is so fast that the caustic mixture, heated to a high temperature, is shot with a loud sound at the enemy. If a jet weighing only half a gram hits human skin, it will cause a slight burn.
The principle “invented” by the beetle is typical for explosives chemical nature, in which energy is released due to the formation of strong chemical bonds. In nuclear weapons, energy is released through fission or fusion atomic nuclei. An explosion is a very rapid release of energy in a limited volume. In this case, instantaneous heating and expansion of the air occurs, and a shock wave begins to spread, leading to great destruction. If you detonate dynamite (without a steel shell) on the Moon, where there is no air, the destructive consequences will be immeasurably less than on Earth. The need for a very rapid release of energy for an explosion is evidenced by this fact. It is well known that a mixture of hydrogen and chlorine explodes if placed on a straight line sunlight or if you bring burning magnesium to the flask - this is written even in school textbooks, but if the light is not so bright, the reaction will take place completely calmly, the same energy will be released in it, but not in a hundredth of a second, but in several hours and as a result, the heat will simply dissipate in the surrounding air.
When any exothermic reaction occurs, the released thermal energy heats not only environment, but also the reagents themselves. This leads to an increase in the rate of reaction, which in turn accelerates the release of heat and this further increases the temperature. If the removal of heat into the surrounding space does not keep pace with its release, then as a result the reaction may, as chemists say, “go wild” - the mixture will boil and splash out of the reaction vessel or even explode if the released gases and vapors do not find a quick exit from the vessel . This is the so-called thermal explosion. Therefore, when carrying out exothermic reactions, chemists carefully monitor the temperature, lowering it if necessary by adding pieces of ice to the flask or placing the vessel in a cooling mixture. It is especially important to be able to calculate the rate of heat release and heat removal for industrial reactors.
Energy is released very quickly in the event of detonation. This word (it comes from the Latin detonare - to thunder) means the chemical transformation of an explosive substance, which is accompanied by the release of energy and the propagation of a wave through the substance at supersonic speed. The chemical reaction is excited by an intense shock wave, which forms the leading front of the detonation wave. The pressure in the shock wave front is tens of thousands of megapascals (hundreds of thousands of atmospheres), which explains the enormous destructive effect of such processes. Energy released in the zone chemical reaction, continuously maintains high pressure in the shock wave. Detonation occurs in many compounds and their mixtures. For example, tetranitromethane C(NO 2) 4 - a heavy colorless liquid with a pungent odor - is distilled without explosion, but its mixtures with many organic compounds detonate with enormous power. Thus, during a lecture at one of the German universities in 1919, many students died due to the explosion of a burner, which was used to demonstrate the combustion of a mixture of tetranitromethane and toluene. It turned out that the laboratory assistant, while preparing the mixture, mixed up the mass and volume fractions of the components, and with reagent densities of 1.64 and 0.87 g/cm3, this caused an almost twofold change in the composition of the mixture, which led to the tragedy.
What substances can explode? First of all, these are the so-called endothermic compounds, that is, compounds the formation of which from simple substances is accompanied not by the release, but by the absorption of energy. Such substances include, in particular, acetylene, ozone, chlorine oxides, peroxides . Thus, the formation of 1 mole of C 2 H 2 from elements is accompanied by a cost of 227 kJ. This means that acetylene should be considered a potentially unstable compound, since the reaction of its decomposition into simple substances C 2 H 2 ® 2C + H 2 is accompanied by the release of very high energy. That is why, unlike many other gases, acetylene is never pumped into cylinders under high pressure - this can lead to an explosion (in cylinders with acetylene, this gas is dissolved in acetone, which is impregnated with a porous carrier).
Acetyleneides of heavy metals - silver, copper - decompose explosively. Pure ozone is also very dangerous for the same reason, the decay of 1 mole of which releases 142 kJ of energy. However, many potentially unstable compounds may turn out to be quite stable in practice. An example is ethylene, the reason for its stability is the very low rate of decomposition into simple substances.
Historically, the first explosive substance invented by people was black (aka black) gunpowder - a mixture of finely ground sulfur, charcoal and potassium nitrate - potassium nitrate (sodium nitrate is not suitable, since it is hygroscopic, that is, it becomes damp in the air). This invention has claimed millions of human lives over the past centuries. However, it turns out that gunpowder was invented for other purposes: the ancient Chinese used gunpowder to create fireworks more than two thousand years ago. The composition of Chinese gunpowder allowed it to burn without exploding.
The ancient Greeks and Romans did not have saltpeter, so they could not have gunpowder. Around the 5th century. saltpeter came from India and China to Byzantium, the capital of the Greek empire. In Byzantium it was discovered that a mixture of saltpeter with flammable substances burns very intensely and cannot be extinguished. Why this happens became known much later - such mixtures do not need air for combustion: saltpeter itself is a source of oxygen). Combustible mixtures containing saltpeter called “Greek fire” began to be used in warfare. With their help, in 670 and 718, the ships of the Arab fleet that besieged Constantinople were burned. In the 10th century Byzantium repelled the Bulgarian invasion with the help of Greek fire.
Centuries have passed, and medieval Europe gunpowder was reinvented. This happened in the 13th century. And who the inventor was is unknown. According to one legend, a monk from Freiburg, Berthold Schwartz, ground a mixture of sulfur, charcoal and saltpeter in a heavy metal mortar. An iron ball accidentally fell into the mortar. There was a terrible roar, acrid smoke poured out of the mortar, and a hole appeared in the ceiling - it was pierced by a ball that flew out of the mortar at great speed. It became clear what enormous power lies in the black powder (the word “gunpowder” itself comes from the Old Russian “dust” - dust, powder). In 1242, gunpowder was described by the English philosopher and naturalist Roger Bacon. Gunpowder began to be used in warfare. In 1300 the first cannon was cast, and soon the first guns appeared. The first gunpowder factory in Europe was built in Bavaria in 1340. In the 14th century. Firearms also began to be used in Rus': with their help, Muscovites defended their city in 1382 from the troops of the Tatar Khan Tokhtamysh.
The invention of gunpowder had a huge impact on world history. With the help of firearms, seas and continents were conquered, civilizations were destroyed, entire nations were destroyed or conquered. But the discovery of gunpowder also had positive aspects. Hunting for wild animals has become easier. In 1627, in Banska Stjavice on the territory of modern Slovakia, gunpowder was first used in mining - to destroy rock in a mine. Thanks to gunpowder special science on calculating the motion of nuclei - ballistics. Methods for casting metals for cannons began to be improved, and new strong alloys were invented and tested. New methods of producing gunpowder were also developed - and above all, saltpeter
The number of gunpowder factories grew all over the world. They were used to produce many types of black powder - for mines, cannons, rifles, including hunting ones. Research has shown that gunpowder has the ability to burn very quickly. The combustion of the most common powder composition is approximately described by the equation 2KNO 3 + S + 3C ® K 2 S + 3CO 2 + N 2 (in addition to sulfide, potassium sulfate K 2 SO 4 is also formed). The specific composition of the products depends on the combustion pressure. D.I. Mendeleev, who studied this issue, pointed out a significant difference in the composition of the solid residue during blank and combat shots.
In any case, when gunpowder burns, a large amount of gases is released. If gunpowder is poured onto the ground and set on fire, it will not explode, but will simply burn quickly, but if it burns in a confined space, for example, in a gun cartridge, then the released gases forcefully push the bullet out of the cartridge, and it flies out of the barrel at high speed. In 1893 on world exhibition in Chicago, the German industrialist Krupp showed a gun that was loaded with 115 kg of black powder; its projectile weighing 115 kg flew over 20 km within 71 seconds, reaching a height of 6.5 km at its highest point
The particles of solids produced by the combustion of black powder create black smoke, and battlefields were sometimes so shrouded in smoke that it obscured the sunlight (in the novel War and Peace described how the smoke made it difficult for commanders to control the course of battles). Particulate matter produced when black powder burns contaminates the bore of firearms, so the barrel of a gun or cannon had to be cleaned regularly.
By the end of the 19th century. black powder has practically exhausted its capabilities. Chemists knew a lot of explosives, but they were not suitable for shooting: their crushing (high explosive) force was such that the barrel would have shattered into pieces even before the shell or bullet left it. This property is possessed, for example, by lead azide Pb(N 3) 2, fulminate of mercury Hg(CNO) 2 - a salt of fulminate (fulmic) acid. These substances explode easily upon friction and impact; they are used to load primers and serve to ignite gunpowder.
In 1884, French engineer Paul Viel invented the new kind gunpowder - pyroxylin. Pyroxylin was obtained back in 1846 by nitration of cellulose (fiber), but for a long time they could not develop a technology for producing gunpowder that was stable and safe to handle. Viel, having dissolved pyroxylin in a mixture of alcohol and ether, obtained a dough-like mass, which, after pressing and drying, gave excellent gunpowder. Lit in air, it burned quietly, and in a cartridge or shell case it exploded with great force from the detonator. The new gunpowder was much more powerful than black gunpowder, and when burned it did not produce smoke, so it was called smokeless. This gunpowder made it possible to reduce the caliber (inner diameter) of shotguns and pistols and thus increase not only the range, but also the accuracy of shooting. In 1889, an even more powerful smokeless gunpowder appeared - nitroglycerin. The great Russian chemist D.I. Mendeleev did a lot to improve smokeless gunpowder. Here's what he himself wrote about it:
“Black smoky gunpowder was found by the Chinese and monks - almost by accident, by touch, by mechanical mixing, in scientific darkness. Smokeless powder was discovered in the full light of modern chemical knowledge. It will amount to new era military affairs, not because it does not allow smoke to obscure the eyes, but mainly because, with less weight, it makes it possible to impart speeds of 600, 800 and even 1000 meters per second to bullets and all other projectiles, and at the same time represents all the makings of further improvements - using scientific research invisible phenomena that occur during its combustion. Smokeless gunpowder constitutes a new link between the power of countries and their scientific development. For this reason, being one of the warriors of Russian science, in my declining years and strength I did not dare to abandon the analysis of the problems of smokeless gunpowder.”
The gunpowder created by Mendeleev successfully passed tests in 1893: it was fired from a 12-inch gun, and naval artillery inspector Admiral Makarov congratulated the scientist on his brilliant victory. With the help of smokeless powder, the firing range was significantly increased. From a huge Big Bertha cannon weighing 750 tons, the Germans fired at Paris from a distance of 128 km. starting speed The speed of the projectile was 2 km/s, and its highest point was located far in the stratosphere at an altitude of 40 km. During the summer of 1918, over 300 shells were fired at Paris, but, of course, this shooting had only psychological significance, since there was no need to talk about any accuracy.
Smokeless powder is used not only in firearms, but also in rocket engines (solid rocket fuel). During the Second World War, our army successfully used solid fuel rockets - they were fired by the legendary Katyusha guards mortars.
The product of phenol nitration, trinitrophenol (picric acid), had a similar fate. It was obtained as early as 1771 and was used as a yellow dye. And only at the end of the 19th century. they began to use it to equip grenades, mines, and shells called lyddita. The colossal destructive power of this substance, used in the Boer War, is vividly described by Louis Boussenard in his adventure novel Captain Rip-Head. And since 1902, safer trinitrotoluene (TNT, Tol) began to be used for the same purposes. Tall is widely used in blasting operations in industry in the form of cast (or pressed) blocks, since this substance can be safely melted when heated above 80 ° C.
Nitroglycerin, which is very dangerous to handle, has the strongest explosive properties. In 1866, Alfred Nobel managed to “tame” it, who, by mixing nitroglycerin with non-flammable material, received dynamite. Dynamite was used to dig tunnels and in many other mining operations. In the first year, its use in the construction of tunnels in Prussia saved 12 million gold marks.
Modern explosives must satisfy many conditions: safety in production and handling, release of large volumes of gases, and efficiency. The cheapest explosive is a mixture of ammonium nitrate and diesel fuel; its production accounts for 80% of all explosives. Which one is the most powerful? It depends on the power criterion. On the one hand, the detonation speed is important, i.e. wave propagation speed. On the other hand, the density of the substance, because the higher it is, the more energy, other things being equal, is released per unit volume. Thus, for the most powerful nitro compounds, both parameters have been improved by 20–25% over more than 100 years, as can be seen from the following table:
Hexogen (1,3,5-trinitro-1,3,5-triazacyclohexane, cyclonite), which in last years became notorious, with the addition of paraffin or wax, as well as in a mixture with other substances (TNT, ammonium nitrate, aluminum) began to be used in 1940. It is used to equip ammunition, and is also part of ammonites used in rock work.
Most powerful explosive, produced (since 1955) on an industrial scale, is HMX (1,3,5,7-tetranitro-1,3,5,7-tetraazocyclooctane). HMX is quite resistant to heat, so it is used for blasting in high-temperature conditions, for example, in deep wells. A mixture of octogen with TNT (octol) is a component of solid rocket fuels. The absolute record is held by hexanitroisowurtzitane, synthesized in the USA in 1990. The shock wave from its explosion travels 30 times faster than sound
Ilya Leenson