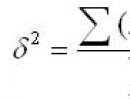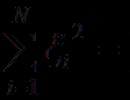Single, double and triple bonds, a- and z-bonds. Chemical bond Double covalent bond examples
chemical bond- these are the interactions of electrons and the atomic nucleus of one particle (atom, ion, molecule, etc.) with electrons and the atomic nucleus of another particle, holding these particles in a stable or metastable chemical compound. The modern description of the chemical bond is carried out on the basis of quantum mechanics. The main characteristics of a chemical bond are strength, length, polarity.
Communication types
- Single electron chemical bond
- metal connection
- covalent bond
- Ionic bond
- Van der Waals connection
- hydrogen bond
- Two-electron three-center chemical bond
The simplest one-electron covalent chemical bond
The simplest one-electron chemical bond is created by a single valence electron. It turns out that one electron is able to hold two positively charged ions in a single whole. In a one-electron bond, the Coulomb repulsive forces of positively charged particles are compensated by the Coulomb forces of attraction of these particles to a negatively charged electron. The valence electron becomes common to the two nuclei of the molecule.
Examples such chemical compounds are molecular ions: H 2+, Li 2+, Na 2+, K 2+, Rb 2+, Cs 2+
Single covalent bond

A single covalent chemical bond is created by a bonding electron pair. In all existing theories (the theory of valence bonds, the theory of molecular orbitals, the theory of repulsion of valence electron pairs, the Bohr model of chemical bonding), the binding electron pair is located in the space between the atoms of the molecule. Distinguish between polar and non-polar covalent bonds.
A nonpolar covalent bond takes place in homonuclear diatomic molecules in which the bonding electronI pair is equidistant from both nuclei of the molecular system.
Distance d between atomic nuclei can be viewed as the sum of the covalent radii of the corresponding atoms.
The distance between atomic nuclei in a single two-electron covalent bond is shorter than the same distance in the simplest one-electron chemical bond.
Multiple covalent bonds
Multiple covalent bonds are represented by unsaturated organic compounds containing double and triple chemical bonds. To describe the nature of unsaturated compounds, L. Pauling introduces the concepts of sigma- and π-bonds, hybridization of atomic orbitals.


Pauling's hybridization for two S- and two p-electrons made it possible to explain the directionality chemical bonds, in particular the tetrahedral configuration of methane. To explain the structure of ethylene, it is necessary to isolate one p-electron from four equivalent Sp3 electrons of the carbon atom to form an additional bond, called the π-bond. In this case, the three remaining Sp2 hybrid orbitals are located in the plane at an angle of 120° and form the main bonds, for example, a planar ethylene molecule.
In the case of the acetylene molecule, only one S and one p orbitals take part in hybridization (according to Pauling), and two Sp orbitals are formed, located at an angle of 180 ° and directed to opposite sides. Two "pure" p-orbitals of carbon atoms overlap in pairs in mutually perpendicular planes, forming two π-bonds of a linear acetylene molecule.
The views of L. Pauling were reflected in his book “The Nature of the Chemical Bond”, which for many years became the reference book of the chemist. In 1954, L. Pauling was awarded Nobel Prize in Chemistry with the wording "For the study of the nature of the chemical bond and its application to the determination of the structure of complex compounds."
However physical meaning selective hybridization of atomic orbitals remained unclear, hybridization represented algebraic transformations to which physical reality could not be attributed.
Linus Pauling made an attempt to improve the description of the chemical bond by eliminating the selectivity of hybridization of orbitals in the molecules of unsaturated compounds and creating the theory of a bent chemical bond. In his report at a symposium on theoretical organic chemistry dedicated to the memory of Kekule (London, September 1958), L. Pauling suggested new way describing a double bond as a combination of two identical bent chemical bonds, and a triple bond as three bent chemical bonds. On this
Symposium L. Pauling categorically stated:
There may be chemists who think that a very important innovation... was the description of the σ,π description for double or triple bonds and conjugated systems, instead of the description by means of bent bonds. I maintain that the σ,π description is less satisfactory than the curved link description, that this innovation is only transitory and will soon die out.
In Pauling's new theory, all binding electrons became equal and equidistant from the line connecting the nuclei of the molecule. Pauling's theory of a curved chemical bond took into account the statistical interpretation of the wave function by M. Born, the Coulomb electron correlation of electrons. A physical meaning appeared - the nature of the chemical bond is completely determined by the electrical interaction of nuclei and electrons. The more bonding electrons, the smaller the internuclear distance and the stronger the chemical bond between carbon atoms.
Three-center chemical bond
Further development of ideas about the chemical bond was given by the American physical chemist W. Lipscomb, who developed the theory of two-electron three-center bonds and a topological theory that makes it possible to predict the structure of some more boron hydrides (borohydrides). 
An electron pair in a three-center chemical bond becomes common to three atomic nuclei. In the simplest representative of a three-center chemical bond - the molecular hydrogen ion H3 +, an electron pair holds three protons in a single whole.
The diborane molecule has four single B-H covalent bonds and two two-electron three-center bonds. The internuclear distance in a single covalent B-H bond is 1.19 Å, while the similar distance in a three-center B-H-B bond is 1.31 Å. The angle of the three-center bond B-H-B (φ) is 830. The combination of two three-center bonds in the diborane molecule makes it possible to keep the nuclei of boron atoms at a distance dB-B = 2 1.31 sin φ/2 = 1.736 Å. The nuclei of the binding hydrogen atoms are removed from the plane in which four single covalent B-H bonds are located, at a distance h = 1.31 · cos φ/2 = 0.981 Å.
Three-center bonds can be realized not only in a triangle of two boron atoms and one hydrogen atom, but also between three boron atoms, for example, in framework borohydrides (pentaborane - B 5 H 9, decaborane - B 10 H 4, etc.). These structures contain ordinary (terminal) and three-center bond (bridge) hydrogen atoms and triangles of boron atoms.
The existence of boranes with their two-electron three-center bonds with "bridge" hydrogen atoms violated the canonical doctrine of valency. The hydrogen atom, previously considered a standard univalent element, turned out to be bound by identical bonds with two boron atoms and became formally a divalent element. The work of W. Lipscomb on deciphering the structure of boranes expanded the understanding of the chemical bond. The Nobel Committee awarded the William Nunn Lipscomb Prize in Chemistry in 1976 with the wording "For his studies of the structure of boranes (borohydrites) which elucidate the problems of chemical bonds".
Multicenter chemical bond
In 1951, T. Keely and P. Pawson unexpectedly obtained a completely new organo-iron compound during the synthesis of dicyclopentadienyl. The preparation of a previously unknown, extremely stable yellow-orange crystalline iron compound immediately attracted attention.

E. Fisher and D. Wilkinson independently established the structure of the new compound - two cyclopentadienyl rings are arranged in parallel, in layers, or in the form of a "sandwich" with an iron atom located between them in the center (Fig. 8). The name "ferrocene" was proposed by R. Woodward (or rather, an employee of his group, D. Whiting). It reflects the presence in the compound of an iron atom and ten carbon atoms (zehn - ten).
All ten bonds (C-Fe) in the ferrocene molecule are equivalent, the Fe-c internuclear distance is 2.04 Å. All carbon atoms in the ferrocene molecule are structurally and chemically equivalent, the length of each C-C bond is 1.40 - 1.41 Å (for comparison, in benzene the C-C bond length is 1.39 Å). A 36-electron shell appears around the iron atom.
In 1973, Ernst Otto Fischer and Jeffrey Wilkinson were awarded the Nobel Prize in Chemistry for their pioneering, independently done work in the field of organometallic, so-called sandwich compounds. Indvar Lindqvist, a member of the Royal Swedish Academy of Sciences, in his speech at the presentation of the laureates, stated that "the discovery and proof of new principles of bonds and structures found in sandwich compounds is a significant achievement, the practical significance of which at present cannot yet be predicted."
At present, dicyclopentadienyl derivatives of many metals have been obtained. Transition metal derivatives have the same structure and the same bond nature as ferrocene. The lanthanides do not form a sandwich structure, but a structure resembling a three-beam star [The atoms of La, Ce, Pr, Nd, therefore, create a fifteen-center chemical bond.
Soon after ferrocene, dibenzenechromium was obtained. Dibenzene-molybdenum and dibenzene-vanadium were prepared according to the same scheme. In all compounds of this class, the metal atoms hold together two six-membered rings. All 12 metal-carbon bonds in these compounds are identical.

Uranocene [bis(cyclooctatetraene)uranium] has also been synthesized, in which the uranium atom holds two eight-membered rings. All 16 uranium-carbon bonds in the uranocene are identical. Uranocene is obtained by reacting UCl 4 with a mixture of cyclooctatetraene and potassium in tetrahydrofuran at minus 300 C.
covalent chemical bond occurs in molecules between atoms due to the formation of common electron pairs. The type of covalent bond can be understood as both the mechanism of its formation and the polarity of the bond. In general, covalent bonds can be classified as follows:
- According to the mechanism of formation, a covalent bond can be formed by an exchange or donor-acceptor mechanism.
- The polarity of a covalent bond can be non-polar or polar.
- According to the multiplicity of the covalent bond, it can be single, double or triple.
This means that a covalent bond in a molecule has three characteristics. For example, in a molecule of hydrogen chloride (HCl), a covalent bond is formed by the exchange mechanism, it is polar and single. In the ammonium cation (NH 4 +), a covalent bond between ammonia (NH 3) and a hydrogen cation (H +) is formed according to the donor-acceptor mechanism, in addition, this bond is polar, is single. In the nitrogen molecule (N 2), the covalent bond is formed by the exchange mechanism, it is non-polar, it is triple.
At exchange mechanism the formation of a covalent bond, each atom has a free electron (or several electrons). Free electrons of different atoms form pairs in the form of a common electron cloud.
At donor-acceptor mechanism the formation of a covalent bond, one atom has a free electron pair, and the other has an empty orbital. The first (donor) gives a pair for common use with the second (acceptor). So in the ammonium cation, nitrogen has a lone pair, and the hydrogen ion has a free orbital.
Non-polar covalent bond formed between atoms of the same chemical element. So in the molecules of hydrogen (H 2), oxygen (O 2), etc., the bond is non-polar. This means that the common electron pair equally belongs to both atoms, since they have the same electronegativity.
Polar covalent bond formed between atoms of different chemical elements. A more electronegative atom displaces an electron pair towards itself. The greater the difference in the electronegativity of the atoms, the more the electrons will be displaced, and the bond will be more polar. So in CH 4, the shift of common electron pairs from hydrogen atoms to carbon atom is not so large, since carbon is not much more electronegative than hydrogen. However, in hydrogen fluoride, the HF bond is highly polar, since the difference in electronegativity between hydrogen and fluorine is significant.
Single covalent bond formed when atoms share the same electron pair double- if two triple- if three. An example of a single covalent bond can be hydrogen molecules (H 2), hydrogen chloride (HCl). An example of a double covalent bond is an oxygen molecule (O 2), where each oxygen atom has two unpaired electrons. An example of a triple covalent bond is a nitrogen molecule (N 2).
Simple (single) bond Types of bonds in bioorganic compounds.
| Parameter name | Meaning |
| Article subject: | Simple (single) bond Types of bonds in bioorganic compounds. |
| Rubric (thematic category) | Chemistry |
covalent bond. Multiple connection. non-polar connection. polar connection.
valence electrons. Hybrid (hybridized) orbital. Link length
Keywords.
Characterization of chemical bonds in bioorganic compounds
AROMATICITY
LECTURE 1
CONNECTED SYSTEMS: ACYCLIC AND CYCLIC.
1. Characterization of chemical bonds in bioorganic compounds. Hybridization of the orbitals of the carbon atom.
2. Classification of conjugate systems: acyclic and cyclic.
3 Types of conjugation: π, π and π, p
4. Criteria for the stability of conjugated systems - ʼʼ conjugation energyʼʼ
5. Acyclic (non-cyclic) conjugate systems, types of conjugation. The main representatives (alkadienes, unsaturated carboxylic acids, vitamin A, carotene, lycopene).
6. Cyclic adjoint systems. Aromatic criteria. Hückel's rule. The role of π-π-, π-ρ-conjugation in the formation of aromatic systems.
7. Carbocyclic aromatic compounds: (benzene, naphthalene, anthracene, phenanthrene, phenol, aniline, benzoic acid) - structure, formation of an aromatic system.
8. Heterocyclic aromatic compounds (pyridine, pyrimidine, pyrrole, purine, imidazole, furan, thiophene) - structure, features of the formation of an aromatic system. Hybridization of electronic orbitals of the nitrogen atom in the formation of five- and six-membered heteroaromatic compounds.
9. Medical biological significance natural compounds containing conjugated bond systems, and aromatic.
The initial level of knowledge for mastering the topic ( school course chemistry):
Electronic configurations of elements (carbon, oxygen, nitrogen, hydrogen, sulfur, halogens), the concept of ʼʼorbitalʼʼ, hybridization of orbitals and spatial orientation of orbitals of elements of period 2., types of chemical bonds, features of the formation of covalent σ- and π-bonds, changes in the electronegativity of elements in a period and group, classification and principles of nomenclature of organic compounds.
Organic molecules are formed through covalent bonds. Covalent bonds arise between two atomic nuclei due to a common (socialized) pair of electrons. This method refers to the exchange mechanism. Non-polar and polar bonds are formed.
Non-polar bonds are characterized by a symmetrical distribution of electron density between the two atoms that this bond connects.
Polar bonds are characterized by an asymmetric (non-uniform) distribution of the electron density, it shifts towards a more electronegative atom.
Electronegativity series (composed downwards)
A) elements: F> O> N> C1> Br> I ~~ S> C> H
B) carbon atom: C (sp) > C (sp 2) > C (sp 3)
Covalent bonds are of two types: sigma (σ) and pi (π).
In organic molecules, sigma (σ) bonds are formed by electrons located on hybrid (hybridized) orbitals, the electron density is located between atoms on the conditional line of their binding.
π-bonds (pi-bonds) arise when two unhybridized p-orbitals overlap. Their main axes are parallel to each other and perpendicular to the σ-bond line. The combination of σ and π bonds is called a double (multiple) bond, it consists of two pairs of electrons. A triple bond consists of three pairs of electrons - one σ - and two π -bonds. (It is extremely rare in bioorganic compounds).
σ - Bonds are involved in the formation of the skeleton of the molecule, they are the main ones, and π -bonds can be considered as additional, but imparting special chemical properties to molecules.
1.2. Hybridization of the orbitals of the carbon atom 6 C
Electronic configuration of the unexcited state of the carbon atom
is expressed by the distribution of electrons 1s 2 2s 2 2p 2 .
At the same time, in bioorganic compounds, as well as in most inorganic substances, the carbon atom has a valency of four.
There is a transition of one of the 2s electrons to a free 2p orbital. Excited states of the carbon atom arise, creating the possibility of the formation of three hybrid states, denoted as С sp 3 , С sp 2 , С sp .
A hybrid orbital has characteristics different from the "pure" s, p, d orbitals and is a "mixture" of two or more types of unhybridized orbitals.
Hybrid orbitals are characteristic of atoms only in molecules.
The concept of hybridization was introduced in 1931 by L. Pauling, Nobel Prize winner.
Consider the arrangement of hybrid orbitals in space.
C sp 3 --- -- -- ---
In the excited state, 4 equivalent hybrid orbitals are formed. The location of the bonds corresponds to the direction of the central angles of a regular tetrahedron, the angle between any two bonds is equal to 109 0 28 , .
In alkanes and their derivatives (alcohols, haloalkanes, amines), all carbon, oxygen, and nitrogen atoms are in the same sp 3 hybrid state. A carbon atom forms four, a nitrogen atom three, an oxygen atom two covalent σ -connections. Around these bonds, the parts of the molecule can freely rotate relative to each other.
In the excited state sp 2, three equivalent hybrid orbitals arise, the electrons located on them form three σ -bonds that are located in the same plane, the angle between the bonds is 120 0 . Unhybridized 2p orbitals of two neighboring atoms form π -connection. It is located perpendicular to the plane in which they are σ -connections. The interaction of p-electrons carries in this case the name ʼʼ lateral overlapʼʼ. A double bond does not allow free rotation of parts of the molecule around itself. The fixed position of the parts of the molecule is accompanied by the formation of two geometric planar isomeric forms, which are called: cis (cis) - and trans (trans) - isomers. (cis- lat- on one side, trans- lat- through).
π -connection
Atoms linked by a double bond are in a state of sp 2 hybridization and
present in alkenes, aromatic compounds, form a carbonyl group
>C=O, azomethine group (imino group) -CH= N-
With sp 2 - --- -- ---
Structural formula an organic compound is represented using Lewis structures (each pair of electrons between atoms is replaced by a dash)
C 2 H 6 CH 3 - CH 3 H H
1.3. Polarization of covalent bonds
A covalent polar bond is characterized by an uneven distribution of electron density. Two conditional images are used to indicate the direction of electron density shift.
Polar σ - bond. The electron density shift is indicated by an arrow along the communication line. The end of the arrow points towards the more electronegative atom. The appearance of partial positive and negative charges is indicated using the letter ʼʼ bʼʼ ʼʼ deltaʼʼ with the desired charge sign.
b + b- b+ b + b- b + b-
CH 3 -\u003e O<- Н СН 3 - >C1 CH 3 -\u003e NH 2
methanol chloromethane aminomethane (methylamine)
Polar π bond. The electron density shift is indicated by a semicircular (curved) arrow above the pi bond, also directed towards the more electronegative atom. ()
b + b- b + b-
H 2 C \u003d O CH 3 - C \u003d== O
methanal |
CH 3 propanone -2
1. Determine the type of hybridization of carbon, oxygen, nitrogen atoms in compounds A, B, C. Name the compounds using the IUPAC nomenclature rules.
A. CH 3 -CH 2 - CH 2 -OH B. CH 2 \u003d CH - CH 2 - CH \u003d O
B. CH 3 - N H - C 2 H 5
2. Make the designations characterizing the direction of polarization of all the indicated bonds in the compounds (A - D)
A. CH 3 - Br B. C 2 H 5 - O- H C. CH 3 -NH- C 2 H 5
G. C 2 H 5 - CH \u003d O
Simple (single) bond Types of bonds in bioorganic compounds. - concept and types. Classification and features of the category "Single (single) bond Types of bonds in bioorganic compounds." 2017, 2018.
Topics USE codifier: Covalent chemical bond, its varieties and mechanisms of formation. Characteristics of a covalent bond (polarity and bond energy). Ionic bond. Metal connection. hydrogen bond
Intramolecular chemical bonds
Let us first consider the bonds that arise between particles within molecules. Such connections are called intramolecular.
chemical bond
between atoms of chemical elements has an electrostatic nature and is formed due to interactions of external (valence) electrons, in more or less degree held by positively charged nuclei bonded atoms.The key concept here is ELECTRONEGNATIVITY. It is she who determines the type of chemical bond between atoms and the properties of this bond.
is the ability of an atom to attract (hold) external(valence) electrons. Electronegativity is determined by the degree of attraction of external electrons to the nucleus and depends mainly on the radius of the atom and the charge of the nucleus.
Electronegativity is difficult to determine unambiguously. L. Pauling compiled a table of relative electronegativity (based on the bond energies of diatomic molecules). The most electronegative element is fluorine with meaning 4 .
It is important to note that in different sources you can find different scales and tables of electronegativity values. This should not be frightened, since the formation of a chemical bond plays a role atoms, and it is approximately the same in any system.
If one of the atoms in the chemical bond A:B attracts electrons more strongly, then the electron pair is shifted towards it. The more electronegativity difference atoms, the more the electron pair is displaced.
If the electronegativity values of the interacting atoms are equal or approximately equal: EO(A)≈EO(V), then the shared electron pair is not displaced to any of the atoms: A: B. Such a connection is called covalent non-polar.
If the electronegativity of the interacting atoms differ, but not much (the difference in electronegativity is approximately from 0.4 to 2: 0,4<ΔЭО<2 ), then the electron pair is shifted to one of the atoms. Such a connection is called covalent polar .
If the electronegativity of the interacting atoms differ significantly (the difference in electronegativity is greater than 2: ΔEO>2), then one of the electrons almost completely passes to another atom, with the formation ions. Such a connection is called ionic.
The main types of chemical bonds are − covalent, ionic and metallic connections. Let's consider them in more detail.
covalent chemical bond
covalent bond – it's a chemical bond formed by formation of a common electron pair A:B . In this case, two atoms overlap atomic orbitals. A covalent bond is formed by the interaction of atoms with a small difference in electronegativity (as a rule, between two non-metals) or atoms of one element.
Basic properties of covalent bonds
- orientation,
- saturability,
- polarity,
- polarizability.
These bond properties affect the chemical and physical properties of substances.
Direction of communication characterizes the chemical structure and form of substances. The angles between two bonds are called bond angles. For example, in a water molecule, the H-O-H bond angle is 104.45 o, so the water molecule is polar, and in the methane molecule, the H-C-H bond angle is 108 o 28 ′.
Saturability is the ability of atoms to form a limited number of covalent chemical bonds. The number of bonds that an atom can form is called.
Polarity bonds arise due to the uneven distribution of electron density between two atoms with different electronegativity. Covalent bonds are divided into polar and non-polar.
Polarizability connections are the ability of bond electrons to be displaced by an external electric field(in particular, the electric field of another particle). The polarizability depends on the electron mobility. The farther the electron is from the nucleus, the more mobile it is, and, accordingly, the molecule is more polarizable.
Covalent non-polar chemical bond
There are 2 types of covalent bonding - POLAR and NON-POLAR .
Example . Consider the structure of the hydrogen molecule H 2 . Each hydrogen atom carries 1 unpaired electron in its outer energy level. To display an atom, we use the Lewis structure - this is a diagram of the structure of the external energy level of an atom, when electrons are denoted by dots. Lewis point structure models are a good help when working with elements of the second period.
H. + . H=H:H
Thus, the hydrogen molecule has one common electron pair and one H–H chemical bond. This electron pair is not displaced to any of the hydrogen atoms, because the electronegativity of hydrogen atoms is the same. Such a connection is called covalent non-polar .
Covalent non-polar (symmetrical) bond - this is a covalent bond formed by atoms with equal electronegativity (as a rule, the same non-metals) and, therefore, with a uniform distribution of electron density between the nuclei of atoms.
The dipole moment of nonpolar bonds is 0.
Examples: H 2 (H-H), O 2 (O=O), S 8 .
Covalent polar chemical bond
covalent polar bond is a covalent bond that occurs between atoms with different electronegativity (usually, different non-metals) and is characterized displacement common electron pair to a more electronegative atom (polarization).
The electron density is shifted to a more electronegative atom - therefore, a partial negative charge (δ-) arises on it, and a partial positive charge arises on a less electronegative atom (δ+, delta +).
The greater the difference in the electronegativity of atoms, the higher polarity connections and even more dipole moment . Between neighboring molecules and charges opposite in sign, additional attractive forces act, which increases strength connections.
Bond polarity affects the physical and chemical properties of compounds. The reaction mechanisms and even the reactivity of neighboring bonds depend on the polarity of the bond. The polarity of a bond often determines polarity of the molecule and thus directly affects such physical properties as boiling point and melting point, solubility in polar solvents.
Examples: HCl, CO 2 , NH 3 .
Mechanisms for the formation of a covalent bond
A covalent chemical bond can occur by 2 mechanisms:
1. exchange mechanism the formation of a covalent chemical bond is when each particle provides one unpaired electron for the formation of a common electron pair:
BUT . + . B= A:B
2. The formation of a covalent bond is such a mechanism in which one of the particles provides an unshared electron pair, and the other particle provides a vacant orbital for this electron pair:
BUT: + B= A:B
In this case, one of the atoms provides an unshared electron pair ( donor), and another atom provides a vacant orbital for this pair ( acceptor). As a result of the formation of a bond, both electron energy decreases, i.e. this is beneficial for the atoms.
A covalent bond formed by the donor-acceptor mechanism, is not different by properties from other covalent bonds formed by the exchange mechanism. The formation of a covalent bond by the donor-acceptor mechanism is typical for atoms either with a large number of electrons in the external energy level (electron donors), or vice versa, with a very small number of electrons (electron acceptors). The valence possibilities of atoms are considered in more detail in the corresponding.
A covalent bond is formed by the donor-acceptor mechanism:
- in a molecule carbon monoxide CO(the bond in the molecule is triple, 2 bonds are formed by the exchange mechanism, one by the donor-acceptor mechanism): C≡O;
- in ammonium ion NH 4 +, in ions organic amines, for example, in the methylammonium ion CH 3 -NH 2 + ;
- in complex compounds, a chemical bond between the central atom and groups of ligands, for example, in sodium tetrahydroxoaluminate Na the bond between aluminum and hydroxide ions;
- in nitric acid and its salts- nitrates: HNO 3, NaNO 3, in some other nitrogen compounds;
- in a molecule ozone O 3 .
Main characteristics of a covalent bond
A covalent bond, as a rule, is formed between the atoms of non-metals. The main characteristics of a covalent bond are length, energy, multiplicity and directivity.
Chemical bond multiplicity
Chemical bond multiplicity - this is the number of shared electron pairs between two atoms in a compound. The multiplicity of the bond can be quite easily determined from the value of the atoms that form the molecule.
For example , in the hydrogen molecule H 2 the bond multiplicity is 1, because each hydrogen has only 1 unpaired electron in the outer energy level, therefore, one common electron pair is formed.
In the oxygen molecule O 2, the bond multiplicity is 2, because each atom has 2 unpaired electrons in its outer energy level: O=O.
In the nitrogen molecule N 2, the bond multiplicity is 3, because between each atom there are 3 unpaired electrons in the outer energy level, and the atoms form 3 common electron pairs N≡N.
Covalent bond length
Chemical bond length
is the distance between the centers of the nuclei of the atoms that form the bond. It is determined by experimental physical methods. The bond length can be estimated approximately, according to the additivity rule, according to which the bond length in the AB molecule is approximately equal to half the sum of the bond lengths in the A 2 and B 2 molecules: 
The length of a chemical bond can be roughly estimated along the radii of atoms, forming a bond, or by the multiplicity of communication if the radii of the atoms are not very different.
With an increase in the radii of the atoms forming a bond, the bond length will increase.
For example
With an increase in the multiplicity of bonds between atoms (whose atomic radii do not differ, or differ slightly), the bond length will decrease.
For example . In the series: C–C, C=C, C≡C, the bond length decreases.
Bond energy
A measure of the strength of a chemical bond is the bond energy. Bond energy is determined by the energy required to break the bond and remove the atoms that form this bond to an infinite distance from each other.
The covalent bond is very durable. Its energy ranges from several tens to several hundreds of kJ/mol. The greater the bond energy, the greater the bond strength, and vice versa.
The strength of a chemical bond depends on the bond length, bond polarity, and bond multiplicity. The longer the chemical bond, the easier it is to break, and the lower the bond energy, the lower its strength. The shorter the chemical bond, the stronger it is, and the greater the bond energy.
For example, in the series of compounds HF, HCl, HBr from left to right the strength of the chemical bond decreases, because the length of the bond increases.
Ionic chemical bond
Ionic bond is a chemical bond based on electrostatic attraction of ions.
ions formed in the process of accepting or giving away electrons by atoms. For example, the atoms of all metals weakly hold the electrons of the outer energy level. Therefore, metal atoms are characterized restorative properties the ability to donate electrons.
Example. The sodium atom contains 1 electron at the 3rd energy level. Easily giving it away, the sodium atom forms a much more stable Na + ion, with the electron configuration of the noble neon gas Ne. The sodium ion contains 11 protons and only 10 electrons, so the total charge of the ion is -10+11 = +1:
+11Na) 2 ) 8 ) 1 - 1e = +11 Na +) 2 ) 8
Example. The chlorine atom has 7 electrons in its outer energy level. To acquire the configuration of a stable inert argon atom Ar, chlorine needs to attach 1 electron. After the attachment of an electron, a stable chlorine ion is formed, consisting of electrons. The total charge of the ion is -1:
+17Cl) 2 ) 8 ) 7 + 1e = +17 Cl — ) 2 ) 8 ) 8
Note:
- The properties of ions are different from the properties of atoms!
- Stable ions can form not only atoms, but also groups of atoms. For example: ammonium ion NH 4 +, sulfate ion SO 4 2-, etc. Chemical bonds formed by such ions are also considered ionic;
- Ionic bonds are usually formed between metals and nonmetals(groups of non-metals);
The resulting ions are attracted due to electrical attraction: Na + Cl -, Na 2 + SO 4 2-.
Let us visually generalize difference between covalent and ionic bond types:
metal chemical bond
metal connection is the relationship that is formed relatively free electrons between metal ions forming a crystal lattice.
The atoms of metals on the outer energy level usually have one to three electrons. The radii of metal atoms, as a rule, are large - therefore, metal atoms, unlike non-metals, quite easily donate outer electrons, i.e. are strong reducing agents
Intermolecular interactions
Separately, it is worth considering the interactions that occur between individual molecules in a substance - intermolecular interactions . Intermolecular interactions are a type of interaction between neutral atoms in which new covalent bonds do not appear. The forces of interaction between molecules were discovered by van der Waals in 1869 and named after him. Van dar Waals forces. Van der Waals forces are divided into orientation, induction and dispersion . The energy of intermolecular interactions is much less than the energy of a chemical bond.
Orientation forces of attraction arise between polar molecules (dipole-dipole interaction). These forces arise between polar molecules. Inductive interactions is the interaction between a polar molecule and a non-polar one. A non-polar molecule is polarized due to the action of a polar one, which gives rise to an additional electrostatic attraction.
A special type of intermolecular interaction is hydrogen bonds. - these are intermolecular (or intramolecular) chemical bonds that arise between molecules in which there are strongly polar covalent bonds - H-F, H-O or H-N. If there are such bonds in the molecule, then between the molecules there will be additional forces of attraction .
Mechanism of education The hydrogen bond is partly electrostatic and partly donor-acceptor. In this case, an atom of a strongly electronegative element (F, O, N) acts as an electron pair donor, and hydrogen atoms connected to these atoms act as an acceptor. Hydrogen bonds are characterized orientation in space and saturation .
The hydrogen bond can be denoted by dots: H ··· O. The greater the electronegativity of an atom connected to hydrogen, and the smaller its size, the stronger the hydrogen bond. It is primarily characteristic of compounds fluorine with hydrogen , as well as to oxygen with hydrogen , less nitrogen with hydrogen .
Hydrogen bonds occur between the following substances:
— hydrogen fluoride HF(gas, solution of hydrogen fluoride in water - hydrofluoric acid), water H 2 O (steam, ice, liquid water):
— solution of ammonia and organic amines- between ammonia and water molecules;
— organic compounds in which O-H or N-H bonds: alcohols, carboxylic acids, amines, amino acids, phenols, aniline and its derivatives, proteins, solutions of carbohydrates - monosaccharides and disaccharides.
The hydrogen bond affects the physical and chemical properties of substances. Thus, the additional attraction between molecules makes it difficult for substances to boil. Substances with hydrogen bonds exhibit an abnormal increase in the boiling point.
For example As a rule, with an increase in molecular weight, an increase in the boiling point of substances is observed. However, in a number of substances H 2 O-H 2 S-H 2 Se-H 2 Te we do not observe a linear change in boiling points.
Namely, at boiling point of water is abnormally high - not less than -61 o C, as the straight line shows us, but much more, +100 o C. This anomaly is explained by the presence of hydrogen bonds between water molecules. Therefore, under normal conditions (0-20 o C), water is liquid by phase state.
covalent bond. Multiple connection. non-polar connection. polar connection.
valence electrons. Hybrid (hybridized) orbital. Link length
Keywords.
Characterization of chemical bonds in bioorganic compounds
AROMATICITY
LECTURE 1
CONNECTED SYSTEMS: ACYCLIC AND CYCLIC.
1. Characterization of chemical bonds in bioorganic compounds. Hybridization of the orbitals of the carbon atom.
2. Classification of conjugate systems: acyclic and cyclic.
3 Types of conjugation: π, π and π, p
4. Criteria for the stability of conjugated systems - "conjugation energy"
5. Acyclic (non-cyclic) conjugate systems, types of conjugation. The main representatives (alkadienes, unsaturated carboxylic acids, vitamin A, carotene, lycopene).
6. Cyclic adjoint systems. Aromatic criteria. Hückel's rule. The role of π-π-, π-ρ-conjugation in the formation of aromatic systems.
7. Carbocyclic aromatic compounds: (benzene, naphthalene, anthracene, phenanthrene, phenol, aniline, benzoic acid) - structure, formation of an aromatic system.
8. Heterocyclic aromatic compounds (pyridine, pyrimidine, pyrrole, purine, imidazole, furan, thiophene) - structure, features of the formation of an aromatic system. Hybridization of electron orbitals of the nitrogen atom in the formation of five- and six-membered heteroaromatic compounds.
9. Medico-biological significance of natural compounds containing conjugated bond systems, and aromatic.
The initial level of knowledge for mastering the topic (school chemistry course):
Electronic configurations of elements (carbon, oxygen, nitrogen, hydrogen, sulfur, halogens), the concept of "orbital", hybridization of orbitals and spatial orientation of the orbitals of elements of the 2nd period., types of chemical bonds, features of the formation of covalent σ- and π-bonds, changes in the electronegativity of elements in period and group, classification and principles of nomenclature of organic compounds.
Organic molecules are formed through covalent bonds. Covalent bonds arise between two atomic nuclei due to a common (socialized) pair of electrons. This method refers to the exchange mechanism. Non-polar and polar bonds are formed.
Non-polar bonds are characterized by a symmetrical distribution of electron density between the two atoms that this bond connects.
Polar bonds are characterized by an asymmetric (non-uniform) distribution of electron density; it shifts towards a more electronegative atom.
Electronegativity series (composed downwards)
A) elements: F> O> N> C1> Br> I ~~ S> C> H
B) carbon atom: C (sp) > C (sp 2) > C (sp 3)
Covalent bonds can be of two types: sigma (σ) and pi (π).
In organic molecules, sigma (σ) bonds are formed by electrons located on hybrid (hybridized) orbitals, the electron density is located between atoms on the conditional line of their binding.
π-bonds (pi-bonds) arise when two unhybridized p-orbitals overlap. Their main axes are parallel to each other and perpendicular to the σ-bond line. The combination of σ and π bonds is called a double (multiple) bond, it consists of two pairs of electrons. A triple bond consists of three pairs of electrons - one σ - and two π -bonds. (It is extremely rare in bioorganic compounds).
σ - Bonds are involved in the formation of the skeleton of the molecule, they are the main ones, and π -bonds can be considered as additional, but imparting special chemical properties to molecules.
1.2. Hybridization of the orbitals of the carbon atom 6 C
Electronic configuration of the unexcited state of the carbon atom
is expressed by the distribution of electrons 1s 2 2s 2 2p 2 .
However, in bioorganic compounds, as well as in most inorganic substances, the carbon atom has a valency of four.
There is a transition of one of the 2s electrons to a free 2p orbital. Excited states of the carbon atom arise, creating the possibility of the formation of three hybrid states, denoted as С sp 3 , С sp 2 , С sp .
A hybrid orbital has characteristics different from the "pure" s, p, d orbitals and is a "mixture" of two or more types of unhybridized orbitals.
Hybrid orbitals are characteristic of atoms only in molecules.
The concept of hybridization was introduced in 1931 by L. Pauling, Nobel Prize winner.
Consider the arrangement of hybrid orbitals in space.
C sp 3 --- -- -- ---
In the excited state, 4 equivalent hybrid orbitals are formed. The location of the bonds corresponds to the direction of the central angles of a regular tetrahedron, the angle between any two bonds is equal to 109 0 28 , .
In alkanes and their derivatives (alcohols, haloalkanes, amines), all carbon, oxygen, and nitrogen atoms are in the same sp 3 hybrid state. A carbon atom forms four, a nitrogen atom three, an oxygen atom two covalent σ -connections. Around these bonds, the parts of the molecule can freely rotate relative to each other.
In the excited state sp 2, three equivalent hybrid orbitals arise, the electrons located on them form three σ -bonds that are located in the same plane, the angle between the bonds is 120 0 . Unhybridized 2p orbitals of two neighboring atoms form π -connection. It is located perpendicular to the plane in which they are σ -connections. The interaction of p-electrons in this case is called "lateral overlap". A double bond does not allow free rotation of parts of the molecule around itself. The fixed position of the parts of the molecule is accompanied by the formation of two geometric planar isomeric forms, which are called: cis (cis) - and trans (trans) - isomers. (cis- lat- on one side, trans- lat- through).
π -connection
Atoms linked by a double bond are in a state of sp 2 hybridization and
present in alkenes, aromatic compounds, form a carbonyl group
>C=O, azomethine group (imino group) -CH= N-
With sp 2 - --- -- ---
The structural formula of an organic compound is depicted using Lewis structures (each pair of electrons between atoms is replaced by a dash)
C 2 H 6 CH 3 - CH 3 H H
1.3. Polarization of covalent bonds
A covalent polar bond is characterized by an uneven distribution of electron density. Two conditional images are used to indicate the direction of electron density shift.
Polar σ - bond. The electron density shift is indicated by an arrow along the communication line. The end of the arrow points towards the more electronegative atom. The appearance of partial positive and negative charges is indicated using the letter "b" "delta" with the desired charge sign.
b + b- b+ b + b- b + b-
CH 3 -\u003e O<- Н СН 3 - >C1 CH 3 -\u003e NH 2
methanol chloromethane aminomethane (methylamine)
Polar π bond. The electron density shift is indicated by a semicircular (curved) arrow above the pi bond, also directed towards the more electronegative atom. ()
b + b- b + b-
H 2 C \u003d O CH 3 - C \u003d== O
methanal |
CH 3 propanone -2
1. Determine the type of hybridization of carbon, oxygen, nitrogen atoms in compounds A, B, C. Name the compounds using the IUPAC nomenclature rules.
A. CH 3 -CH 2 - CH 2 -OH B. CH 2 \u003d CH - CH 2 - CH \u003d O
B. CH 3 - N H - C 2 H 5
2. Make the designations characterizing the direction of polarization of all the indicated bonds in the compounds (A - D)
A. CH 3 - Br B. C 2 H 5 - O- H C. CH 3 -NH- C 2 H 5