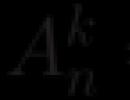Experience of thermal conductivity of water and a metal spoon. Study of the thermal conductivity of various substances. IV. Helpful Hints
Lesson topic:Entertaining physics lesson
on the topic "thermal phenomena"
Lesson Objectives:
1. Educational: to systematize students' knowledge on the topic "Thermal phenomena" and demonstrate to students entertaining experiments using home-made equipment.
2. Nurturing:
3. Developing: develop logic, clarity and brevity of speech, physical terminology, generalization skills, general erudition of students.
Equipment:
Demos:
Lesson plan
Organizing time
Setting the goal of the lesson
Knowledge update
Demonstration of entertaining experiments and their explanation based on the material covered earlier
Homework
Lesson summary
During the classes
Organizing time
Setting the goal of the lesson
For several lessons, we have considered various thermal processes and learned to explain them on the basis of modern knowledge of physics.
Today in the lesson we will look at a number of entertaining experiments on this topic and explain what we observe based on the knowledge we have.
Knowledge update
But from the beginning, let's recall the material we studied earlier.
Questions:
What are the thermal phenomena?
Give examples of thermal phenomena?
What characterizes the temperature?
How is the temperature of a body related to the speed of movement of its molecules?
What is the difference between the movement of molecules in gases, liquids and solids?
Demonstration of entertaining experiments
Physics around us! We meet her everywhere. And what experiments can be carried out at home without using expensive instruments and equipment? Very simple and entertaining...
Experiment #1
"Focus for New Year's Eve"
This trick is best shown on New Year's Eve in a room lit only by a Christmas tree garland. The magician takes two candles from the table. He connects them with wicks, pronounces a "magic spell" - and now ... smoke appears at the point of contact of the wicks, followed by fire. The magician spreads candles to the sides - they burn! What is the secret of focus?
Answer: Those who are fond of chemistry have probably already figured out what the secret of the trick is in a self-igniting mixture. Before demonstrating the trick, prepare the props, for this you need to sprinkle the wick of one of the candles with potassium permanganate powder (potassium permanganate), and soak the other with liquid glycerin. Remember, ignition does not occur immediately, it takes some time. Be careful. The fire is real.
Experiment #2
"BOILER"
Can water boil at room temperature?
To answer this question, we will conduct the following experiment: I filled a disposable medical syringe, in which there was no needle, by 1/8 with water. Then close the hole with your finger and sharply pull the piston to its extreme position. The water inside the syringe "boiled", remaining cold. Why does water "boil"?
Answer: The boiling point depends on pressure. The lower the gas pressure above the liquid surface, the lower the boiling point of this liquid.
Experiment #3
"Can not be?"
For an experiment, boil a hard-boiled egg.
Peel it off the shell. Take a piece of paper
80 by 80 mm, roll it up like an accordion and set it on fire. Then dip the burning paper into a wide-mouthed bottle.
After 1-2 seconds, cover the neck with an egg (see figure). The burning of the paper stops, and the egg begins to be drawn into the decanter. Explain the observed phenomenon.
Answer: When the paper burns, the air inside the bottle heats up and expands. When the flame went out, the air in the bottle cooled and, accordingly, its pressure decreased, and atmospheric pressure pushed the egg into the bottle.
Comment: This experience can be made more interesting by inserting an incompletely peeled banana into the neck of the bottle. Being drawn into the bottle, he will be cleansed at the same time
Experiment #4
"Crawling Glass"
Take a clean window glass about 30 - 40 cm long. Place two matchboxes under one edge of the glass so that an inclined plane is formed. Moisten the rim of a glass of thin glass with water and place upside down on the glass. Bring a burning candle to the wall of the glass and the glass will slowly crawl. How to explain it?
Answer: This is due to the fact that when heated, the air inside the glass expands and slightly raises the glass. Water prevents air from escaping from the glass, as a result, the friction force between the glass and the glass decreases and the glass creeps down.
Experiment #5
"Observation of Evaporation and Condensation"

Experiment #6
Observe convection in cold and hot water using potassium permanganate crystals, a drop of brilliant green, or any other coloring matter as a dye. Compare the nature and speed of convection and draw conclusions
Experiment #7

It's interesting that...
The longest in history scientific research The experiment is taking place at a university in Australia. Back in 1927, the first dean of the Faculty of Physics of this university, T. Parnell, melted some bitumen, poured it into a funnel with a stopper at the end, let it cool and settle for three years, and then took out the stopper. Since then, on average, once every 9 years, a drop of resin falls from the funnel into a glass placed below. The last drop fell at Christmas in 1999. It is believed that the funnel will be empty not earlier than in another 100 years.
PEOPLE'S WISDOM
Proverbs:
"A lot of snow - a lot of bread" Why?
Answer: Snow has poor thermal conductivity, i.e. snow is a "fur coat" for the earth, it keeps it warm. The fur coat is thick, the frost will not get to the winter crops, it will protect them from freezing.
"Without a lid, the samovar does not boil; without a mother, a child cannot frolic." Why doesn't a samovar boil for a long time without a lid?
Answer: With the lid open, some of the molecules with high kinetic energy will fly away from the surface of the water, taking energy with them.
"Frozen - as at the bottom of the sea." Why is it always cold on the seabed?
Answer: The sun's rays do not warm up the deep layers of water: thermal, infrared rays - are absorbed by almost all the water surface. In addition, water has a relatively low thermal conductivity.
Tasks - riddles
In winter it warms, in spring it smolders, in summer it dies, in autumn it flies.(Snow.)
The world warms, does not know fatigue.(The sun.)
How does the Sun's energy reach the Earth?
Answer.radiation. (by electromagnetic waves)
Hanging pear - you can not eat; do not be afraid - touch, even though there is fire inside.(Electric lamp.)
Runs without legs, burns without fire.(Electricity.)
As the Sun burns, it flies faster than the wind, the road lies in the air, it has no equal in strength.(Lightning.)
Who, without learning, speaks all languages?(Echo.)
He walks along the sea, he walks, and when he reaches the shore, he will disappear there.(Wave.)
Curls around the nose, but not in the hands.(Smell.)
Without wings, without a body, she flew a thousand miles away.(Radio wave. )
How can you carry water in a sieve?(Freezing water.)
Homework
Prepare ice in the freezer. Fold it in a plastic bag and wrap it with a downy scarf or cover it with cotton wool. Can be additionally wrapped in a fur coat. Leave this package for 5-7 hours, then check the ice. Explain the observed state.
Suggest at home a way to preserve frozen food when defrosting the refrigerator.
Lesson summary
Today in the lesson we remembered what thermal phenomena are, observed examples of thermal phenomena in experiments set up using elementary, improvised equipment and explained these phenomena.
Summing up the lesson, grading.
Sections: Physics
The purpose of the work is a generalization of experimental tasks carried out by students of the 8th grade at home in the study of various types of heat transfer.
Tasks:
- Explore additional literature on the topic "Types of heat transfer".
- Conduct experiments at home.
- Analyze and summarize the results of experiments. Compare your results with the conclusions suggested in the textbook.
- Give additional examples from life (not including materials from the educational material).
- Develop recommendations "Useful tips" using the conclusions of the topic "Types of heat transfer".
I. Experiments on thermal conductivity.
- Pour the same amount of hot water into glass and aluminum glasses of the same mass and the same capacity at the same time. Touching the glasses with your hand will show that the aluminum glass warms up faster, this is because the thermal conductivity of aluminum is higher than the thermal conductivity of glass.
- Pour tea into aluminum and porcelain mugs. When we drink tea from an aluminum mug, we will burn our lips more than from porcelain, because when we touch the mug with our lips and thereby cool some of its area, large quantity The heat from the hot tea is transferred to the lips through the aluminum mug, as the thermal conductivity of aluminum is higher than that of porcelain.
- We prick a number of buttons on a wooden cylinder or bar (you can depict some figure from them). We wrap a bar or cylinder with one layer of paper and place it in a candle flame for a short time. There is an uneven charring of the paper, less in places where the paper touches the buttons, due to the fact that the thermal conductivity of metal is higher than that of wood.
- We wrap the room thermometer in a fur coat and check if its readings change after a while. Of course, this does not happen, having demonstrated this experiment to parents, we explain why the fur coat does not warm. (The fur coat itself cannot heat, since it is not a source of energy itself, it is only a heat insulator, preventing us from freezing in winter, moreover, there is an air layer between the human body and the fur coat).
In order to better understand the essence of the phenomenon of heat conduction, it is necessary to explain the following phenomena:
but) Why do metal objects seem colder than wooden objects at the same temperature?
Answer: Wood has poor thermal conductivity, so when we touch a wooden object, only a small area of the body under the arm heats up. The metal also has good thermal conductivity, so when in contact with the hand, a much larger area heats up. This leads to greater heat dissipation from the hand and its cooling.
b) Why are the handles of faucets and hot water tanks made of wood or plastic?
Answer: wood and plastic have poor thermal conductivity.
in) Does ordinary or porous brick provide the best thermal insulation of the building?
Answer: Porous brick contains air in its pores, which has poor thermal conductivity, so it provides better thermal insulation of the building.
G) Is air used as a building material?
Answer: Yes, it is used, because foam materials, porous bricks, glass wool contain air that has poor thermal conductivity.
e) depending on how much volume the pores of the foam occupy, its density is different. Does the thermal conductivity of foam plastic depend on its density?
Answer: The lower the density of the foam, the more pores occupied by air with poor thermal conductivity. Therefore, the lower the density of the foam, the lower its thermal conductivity.
g) why insert double frames?
h) Why do birds often freeze in flight?
Answer: In frost, the birds sit ruffled, which creates an airy shell around their body. During flight, the air near the body of a bird changes all the time, taking away heat.
II. Convection experiments.
- The cooling of the pan with hot liquid was carried out in two ways: 1 - the pan was placed on ice and 2 - ice was placed on the pan.
In the second case, cooling was faster. This is explained as follows. When we put ice on a pan, the top layers cool and become heavier, causing them to sink down. In their place come more heated layers of liquid. Thus, as a result of convection, the liquid is cooled. In the second case, convection will not occur, because. cooling will occur from below, and the cold layers cannot rise up, the cooling process will be slow, the liquid will not be mixed. Thus, we can offer parents to cool any food from above: put them not on ice, but on top of ice, because they are cooled not so much by ice as by cold air that descends. - The rate of natural mixing of water was determined in two cases: 1 - cold water is poured into hot water and 2 - hot water is poured into cold water. For this experiment, you need a stopwatch or watch with a second hand and a thermometer. The volumes of cold and hot water must be taken equal. The thermometer controls the steady temperature, and the stopwatch or clock controls the time. The rate of temperature equalization will be faster when cold water is poured into hot water, since hot water will rise up, and cold water will fall down. Thus, mixing will occur quickly and evenly. This means that the temperature will even out faster.
- A lit candle is covered with a glass cylindrical tube, while the flame decreases and may go out, because. combustion occurs in the presence of oxygen, and in this experiment, convection phenomena cannot occur, there is no air inflow. If you raise the tube, the candle will burn brighter. If, however, the tube is not lifted, but a paper partition is lowered into it, not reaching the flame, then it will increase. In this case, cold air will descend along the paper, displacing the heated air, in which there is little oxygen, thereby increasing the flow of oxygen to the flame.
- In A.S. Pushkin's poem "The Caucasus" there are such lines: "The eagle, having risen from a distant peak, soars motionless on a par with me." The phenomenon that large birds can soar in the air, keeping at the same height, without flapping their wings, is explained by the fact that the air heated near the ground rises to a considerable height, these warm streams keep the bird with outstretched wings in the air.
In addition to these experimental tasks, the following questions were answered:
but) Why does it blow from a tightly closed window in cold weather?
Answer: Glass has a lower temperature than the temperature in the room. The air near the glass cools down and sinks down as denser air, then heats up near the radiator and again moves around the room. This movement of air is felt near the window.
b) Where is the best place to place the vent?
Answer: the window is best placed at the top of the window. Warm air is lighter, it is located in the upper part of the room, it will be replaced by colder air from the street. With this arrangement of the window, the room will be ventilated more quickly.
in) when is the draft in the pipe better - in winter or summer?
Answer: draft will be better in winter, when the difference between the temperature of the air heated in the pipe and the outside will be greater, then the pressure drop at the top and bottom of the pipe will be more significant.
G) What role does convection play in heating water in a kettle?
Answer: heated layers of water, as lighter ones, rise up, giving way to cold ones. Thus, due to the movement of convection currents, all the water in the kettle is heated.
e) why does the lampshade or ceiling turn black above incandescent lamps?
Answer: Convection currents of air rise from incandescent lamps, carrying dust particles with them, which then settle on the lampshade or ceiling.
e) why do aspen leaves sway even in calm weather?
Answer: compared to other trees, aspen leaves have long and thin stems. There are vertical convection currents above the ground even in calm weather. Due to its structure, aspen leaves are sensitive to any, even minor fluctuations in the air.
g) can you keep ice cream with a fan?
Answer: No, you can't, because the air flow coming from the fan will always carry away the cold air that forms around the ice cream, thereby accelerating the air exchange process, and the ice cream will melt faster.
h) What natural phenomena occur due to convection?
Answer: winds blowing in the earth's atmosphere; the existence of warm and cold sea currents, mountain building processes.
III. Radiation experiments.
- We take a glass that has edges. We glue the edges of the glass from the inside with strips of white and black paper. We set the candle in the glass so that it stands in the center of the glass (you can center it using cardboard circles with a hole in the center). Glue button caps to each strip of paper with plasticine. The wick of the candle should not reach the edge of the glass a little. After the candle is lit, we observe that the buttons will begin to fly off the black stripes. Experience illustrates that the white color reflects the rays falling on it, and the black absorbs them, so the black edges heated up faster and the buttons peeled off from them in the first place.
To understand this phenomenon, the following questions were answered:
but) Why does snow melt faster in the city than outside the city?
Answer: snow in the city is dirtier, so it absorbs energy better and melts
b) In which of the two vessels will water boil faster in light or smoky?
Answer: In smoky, because. this surface will absorb energy better.
in) Why is a thermos flask mirrored?
Answer: to avoid heating by radiant energy.
IV. Helpful Hints.
- Cooling of food is faster if the cold source is placed at the top, and not at the bottom.
- For the fastest cooling of coffee or tea, you need to pour cold milk into a hot drink.
- Window frames need to be closed more tightly both inside and out. Then the heat loss will be less.
- In severe frost, under a fur coat it is better to wear not one thick sweater, but "multi-layered" clothes.
- If you need to quickly melt snow or ice, it must be sprinkled with dark powder or ash.
- In the hot season it is better to wear light-colored clothes.
- It is safer to use porcelain mugs than aluminum ones.
Conclusion.
The phenomena that we constantly encounter in everyday life were studied not only in the classroom, but also at home, where students could demonstrate them to their parents. These experiments, questions helped to better understand the topic "Types of heat transfer". Analysis of the results made it possible to offer "Useful Tips" It should be noted that all experimental work must be carried out very carefully, in compliance with safety regulations.
Literature.
- A.A. Peryshkin. Physics. textbook for grade 8. Bustard, M. 2004
- Cl. E. Swartz. Extraordinary physics of ordinary phenomena. Science, M. 1986
- A.V. Aganov, R.K. Safiullin, A.I. Skvortsov, D.A. Tayursky. Physics around us. "House of Pedagogy", M. 1998
- Physics. Independent and test papers in physics for grade 8. "Ileksa", M. 2006
- Yu.G. Pavlenko. Beginnings of physics. "Exam", M. 2005
In this lesson, the concept of thermal conductivity is considered.
Thermal conductivity is one of the types of heat transfer and is associated with the transfer of internal energy from more heated parts of the body (bodies) to less heated ones, which is carried out by randomly moving particles of the body.
Each of us encounters thermal conductivity when we inadvertently grab the iron handle of a frying pan on the stove. The poor thermal conductivity of the air makes it possible to insulate the apartment for the winter with the help of double frames. And there are many such examples. Therefore, thermal conductivity is one of the most important physical thermal phenomena that we will study.
In the last lesson, we found out that heat transfer (Fig. 1) can be of three types: conduction, convection and radiation(Fig. 2). In this lesson, we will take a closer look at the first type of heat transfer, namely thermal conductivity.
Rice. 1. Heat transfer

Rice. 2 Types of heat transfer
Thermal conductivity is characteristic of substances in all three states of aggregation: solid, liquid and gaseous (Fig. 3).

Rice. 3. Thermal conductivity is characteristic of all states of aggregation
At the same time, they have the highest thermal conductivity solid bodies(metals) (Fig. 4a), and the lowest - gases (Fig. 4b).

Rice. 4 Thermal conductivity coefficients of various substances
Thermal conductivity is associated with the internal structure of bodies and depends on the arrangement of molecules, their movement and interaction with each other (Fig. 5).

Rice. 5. Connection of thermal conductivity with the internal structure of bodies
It is important to note that during heat conduction, there is no transfer of matter, but there is a transfer of energy from particle to particle or from one body to another during their direct contact. Let us formulate, in fact, the definition of thermal conductivity.
Definition.Thermal conductivity- This is a phenomenon in which energy is transferred from one part of the body to another through the collision of particles or through direct contact between two bodies.

Rice. 6. Illustration of the definition of thermal conductivity
Studies of this phenomenon were carried out mainly empirically. The first experiments on the study of this phenomenon were carried out, apparently, by Galileo Galilei (Fig. 7).

Rice. 7. Galileo Galilei (1564-1642)
The essence of his experiments was simple: Galileo placed various bodies near his thermoscope (Fig. 8) and observed the change in temperature. Subsequently, he drew conclusions: whether bodies conduct heat well or not.

Fig 8. Galileo's thermoscope
Definition.Thermal Conduction Process- is the process of energy transfer from one particle to another, located in close proximity to each other (Fig. 9).

Rice. 9. Heat conduction process
Metals have higher thermal conductivity, since the particles are located close to each other (Fig. 10).

Rice. 10. Thermal conductivity in metals
In liquids, although the molecules are closely spaced, they are quite well isolated (Fig. 11).

Rice. 11. Thermal conductivity in liquids
Gases have the lowest thermal conductivity: molecules are located far from each other, and in order to transfer energy, they need to collide, so the process of energy transfer is rather slow (Fig. 12).

Rice. 12. Thermal conductivity in gases
Consider an experiment that clearly demonstrates the thermal conductivity of metals.
An aluminum rod is fixed horizontally on a tripod. Wooden toothpicks are vertically fixed with wax on the rod at regular intervals. A candle is brought to the edge of the rod (Fig. 13).
Since the edge of the rod heats up, and aluminum, like any other metals, has a fairly good thermal conductivity, the rod gradually warms up. When heat reaches the place where the toothpick is attached to the core, the stearin melts and the toothpick falls off.

Rice. 13. Demonstration of experience
We see that in this experiment there is no transfer of matter, respectively, thermal conductivity is observed.
We have considered the phenomenon of thermal conductivity, and in conclusion we would like to recall an important fact: no particles - no thermal conductivity.
In the next lesson, we will take a closer look at another type of heat transfer - convection.
Bibliography
- Gendenstein L.E., Kaidalov A.B., Kozhevnikov V.B. / Ed. Orlova V.A., Roizena I.I. Physics 8. - M.: Mnemosyne.
- Peryshkin A.V. Physics 8. - M.: Bustard, 2010.
- Fadeeva A.A., Zasov A.V., Kiselev D.F. Physics 8. - M.: Enlightenment.
- Internet portal "experiment.edu.ru" ()
- Internet portal "festival.1september.ru" ()
- Internet portal "class-fizika.narod.ru" ()
Homework
- Page 13, paragraph 4, questions 1-6, exercise 1 (1-3). Peryshkin A.V. Physics 8. - M.: Bustard, 2010.
- Why do gases have low thermal conductivity?
- Why does the water cool down more slowly in an old kettle, after it is removed from the fire, than in the same new one?
- What are double window frames for?
- Why do residents Central Asia during the heat wear wadded bathrobes and hats?
Sections: Physics
The purpose of the work is a generalization of experimental tasks carried out by students of the 8th grade at home in the study of various types of heat transfer.
Tasks:
- To study additional literature on the topic "Types of heat transfer".
- Conduct experiments at home.
- Analyze and summarize the results of experiments. Compare your results with the conclusions suggested in the textbook.
- Give additional examples from life (not including materials from the educational material).
- Develop recommendations "Useful tips" using the conclusions of the topic "Types of heat transfer".
I. Experiments on thermal conductivity.
- Pour the same amount of hot water into glass and aluminum glasses of the same mass and the same capacity at the same time. Touching the glasses with your hand will show that the aluminum glass warms up faster, this is because the thermal conductivity of aluminum is higher than the thermal conductivity of glass.
- Pour tea into aluminum and porcelain mugs. When we drink tea from an aluminum mug, we will burn our lips more than from a porcelain mug, because when we touch the mug with our lips and thereby cool some of its area, more heat from hot tea is transferred to the lips through the aluminum mug, since the thermal conductivity of aluminum higher than porcelain.
- We prick a number of buttons on a wooden cylinder or bar (you can depict some figure from them). We wrap a bar or cylinder with one layer of paper and place it in a candle flame for a short time. There is an uneven charring of the paper, less in places where the paper touches the buttons, due to the fact that the thermal conductivity of metal is higher than that of wood.
- We wrap the room thermometer in a fur coat and check if its readings change after a while. Of course, this does not happen, having demonstrated this experiment to parents, we explain why the fur coat does not warm. (The fur coat itself cannot heat, since it is not a source of energy itself, it is only a heat insulator, preventing us from freezing in winter, moreover, there is an air layer between the human body and the fur coat).
In order to better understand the essence of the phenomenon of heat conduction, it is necessary to explain the following phenomena:
but) Why do metal objects seem colder than wooden objects at the same temperature?
Answer: Wood has poor thermal conductivity, so when we touch a wooden object, only a small area of the body under the arm heats up. The metal also has good thermal conductivity, so when in contact with the hand, a much larger area heats up. This leads to greater heat dissipation from the hand and its cooling.
b) Why are the handles of faucets and hot water tanks made of wood or plastic?
Answer: wood and plastic have poor thermal conductivity.
in) Does ordinary or porous brick provide the best thermal insulation of the building?
Answer: Porous brick contains air in its pores, which has poor thermal conductivity, so it provides better thermal insulation of the building.
G) Is air used as a building material?
Answer: Yes, it is used, because foam materials, porous bricks, glass wool contain air that has poor thermal conductivity.
e) depending on how much volume the pores of the foam occupy, its density is different. Does the thermal conductivity of foam plastic depend on its density?
Answer: The lower the density of the foam, the more pores occupied by air with poor thermal conductivity. Therefore, the lower the density of the foam, the lower its thermal conductivity.
g) why insert double frames?
h) Why do birds often freeze in flight?
Answer: In frost, the birds sit ruffled, which creates an airy shell around their body. During flight, the air near the body of a bird changes all the time, taking away heat.
II. Convection experiments.
- The cooling of the pan with hot liquid was carried out in two ways: 1 - the pan was placed on ice and 2 - ice was placed on the pan.
In the second case, cooling was faster. This is explained as follows. When we put ice on a pan, the top layers cool and become heavier, causing them to sink down. In their place come more heated layers of liquid. Thus, as a result of convection, the liquid is cooled. In the second case, convection will not occur, because. cooling will occur from below, and the cold layers cannot rise up, the cooling process will be slow, the liquid will not be mixed. Thus, we can offer parents to cool any food from above: put them not on ice, but on top of ice, because they are cooled not so much by ice as by cold air that descends. - The rate of natural mixing of water was determined in two cases: 1 - cold water is poured into hot water and 2 - hot water is poured into cold water. For this experiment, you need a stopwatch or watch with a second hand and a thermometer. The volumes of cold and hot water must be taken equal. The thermometer controls the steady temperature, and the stopwatch or clock controls the time. The rate of temperature equalization will be faster when cold water is poured into hot water, since hot water will rise up, and cold water will fall down. Thus, mixing will occur quickly and evenly. This means that the temperature will even out faster.
- A lit candle is covered with a glass cylindrical tube, while the flame decreases and may go out, because. combustion occurs in the presence of oxygen, and in this experiment, convection phenomena cannot occur, there is no air inflow. If you raise the tube, the candle will burn brighter. If, however, the tube is not lifted, but a paper partition is lowered into it, not reaching the flame, then it will increase. In this case, cold air will descend along the paper, displacing the heated air, in which there is little oxygen, thereby increasing the flow of oxygen to the flame.
- In A.S. Pushkin's poem "The Caucasus" there are such lines: "The eagle, having risen from a distant peak, soars motionless on a par with me." The phenomenon that large birds can soar in the air, keeping at the same height, without flapping their wings, is explained by the fact that the air heated near the ground rises to a considerable height, these warm streams keep the bird with outstretched wings in the air.
In addition to these experimental tasks, the following questions were answered:
but) Why does it blow from a tightly closed window in cold weather?
Answer: Glass has a lower temperature than the temperature in the room. The air near the glass cools down and sinks down as denser air, then heats up near the radiator and again moves around the room. This movement of air is felt near the window.
b) Where is the best place to place the vent?
Answer: the window is best placed at the top of the window. Warm air is lighter, it is located in the upper part of the room, it will be replaced by colder air from the street. With this arrangement of the window, the room will be ventilated more quickly.
in) when is the draft in the pipe better - in winter or summer?
Answer: draft will be better in winter, when the difference between the temperature of the air heated in the pipe and the outside will be greater, then the pressure drop at the top and bottom of the pipe will be more significant.
G) What role does convection play in heating water in a kettle?
Answer: heated layers of water, as lighter ones, rise up, giving way to cold ones. Thus, due to the movement of convection currents, all the water in the kettle is heated.
e) why does the lampshade or ceiling turn black above incandescent lamps?
Answer: Convection currents of air rise from incandescent lamps, carrying dust particles with them, which then settle on the lampshade or ceiling.
e) why do aspen leaves sway even in calm weather?
Answer: compared to other trees, aspen leaves have long and thin stems. There are vertical convection currents above the ground even in calm weather. Due to its structure, aspen leaves are sensitive to any, even minor fluctuations in the air.
g) can you keep ice cream with a fan?
Answer: No, you can't, because the air flow coming from the fan will always carry away the cold air that forms around the ice cream, thereby accelerating the air exchange process, and the ice cream will melt faster.
h) What natural phenomena occur due to convection?
Answer: winds blowing in the earth's atmosphere; the existence of warm and cold sea currents, mountain building processes.
III. Radiation experiments.
- We take a glass that has edges. We glue the edges of the glass from the inside with strips of white and black paper. We set the candle in the glass so that it stands in the center of the glass (you can center it using cardboard circles with a hole in the center). Glue button caps to each strip of paper with plasticine. The wick of the candle should not reach the edge of the glass a little. After the candle is lit, we observe that the buttons will begin to fly off the black stripes. Experience illustrates that the white color reflects the rays falling on it, and the black absorbs them, so the black edges heated up faster and the buttons peeled off from them in the first place.
To understand this phenomenon, the following questions were answered:
but) Why does snow melt faster in the city than outside the city?
Answer: snow in the city is dirtier, so it absorbs energy better and melts
b) In which of the two vessels will water boil faster in light or smoky?
Answer: In smoky, because. this surface will absorb energy better.
in) Why is a thermos flask mirrored?
Answer: to avoid heating by radiant energy.
IV. Useful tips.
- Cooling of food is faster if the cold source is placed at the top, and not at the bottom.
- For the fastest cooling of coffee or tea, you need to pour cold milk into a hot drink.
- Window frames need to be closed more tightly both inside and out. Then the heat loss will be less.
- In severe frost, under a fur coat it is better to wear not one thick sweater, but "multi-layered" clothes.
- If you need to quickly melt snow or ice, it must be sprinkled with dark powder or ash.
- In the hot season it is better to wear light-colored clothes.
- It is safer to use porcelain mugs than aluminum ones.
Conclusion.
The phenomena that we constantly encounter in everyday life were studied not only in the classroom, but also at home, where students could demonstrate them to their parents. These experiments, questions helped to better understand the topic "Types of heat transfer". Analysis of the results made it possible to offer "Useful Tips" It should be noted that all experimental work must be carried out very carefully, in compliance with safety regulations.
Literature.
- A.A. Peryshkin. Physics. textbook for grade 8. Bustard, M. 2004
- Cl. E. Swartz. Extraordinary physics of ordinary phenomena. Science, M. 1986
- A.V. Aganov, R.K. Safiullin, A.I. Skvortsov, D.A. Tayursky. Physics around us. "House of Pedagogy", M. 1998
- Physics. Independent and control work in physics for grade 8. "Ileksa", M. 2006
- Yu.G. Pavlenko. Beginnings of physics. "Exam", M. 2005
Option 1. Equipment: A test tube with water and an alcohol lamp.
To demonstrate the poor thermal conductivity of the liquid, water is poured into the test tube ¾ of the volume. Holding the test tube in your hands at a slight angle above the flame of the spirit lamp, heat the water at the open end (Fig. 130). They show that the water here boils quickly, but at the bottom there is no great heating.


Rice. 130 Fig. 2.105 Fig. 131
Experience 4. Thermal conductivity of gases
Option 1. Equipment: two test tubes, two stoppers, two rods, two balls, a spirit lamp, a tripod, a hanger.
Poor thermal conductivity of air is demonstrated using two identical tubes closed with stoppers through which short rods are passed. Steel balls are attached to the ends of the rods with plasticine or paraffin (Fig. 131). The test tubes above the alcohol lamp are arranged so that convection occurs in one of them, and the thermal conductivity of air occurs in the other. It is noticed that in one test tube the ball quickly falls away from the rod.
Option 2. See fig. 2.105
Experience 5. Convection of liquids
Option 1. Equipment: a device for demonstrating the convection of a liquid, potassium permanganate, a spirit lamp, a tripod.
The device, which is a closed glass tube (Fig. 132), is fixed in the foot of the tripod. (It is better to hang the tube than to clamp it at the bottom, for in the latter case the glass is more likely to break.) Fill the tube with water through the upper opening of any elbow so that there are no air bubbles along the entire closed path inside the tube.
When performing the experiment, potassium permanganate crystals are placed in a spoon with a grid and lowered into the knee (you can simultaneously lower two spoons with potassium permanganate crystals into both knees). Then a spirit lamp is brought to the lower part of this knee and convection is observed.



Rice. 132 Fig. 133
Experience 6. Convection of gases
Option 1. Equipment: spirit lamp, matches, paper snake, metal point.
To demonstrate gas convection, a paper snake is made, which rotates in a stream of ascending hot air coming from a spirit stove or electric stove (Fig. 133). (When installing the snake on the point, do not pierce the paper.)
Experience 7. Heating by radiation
Option 1. Equipment: heat sink, open demonstration pressure gauge, table lamp (or electric stove).
The heat sink, connected by a tube to a demonstration manometer (see Fig. 123), is fixed in a tripod opposite the radiator. As a radiating body, you can take an electric stove, a vessel with hot water, etc. A heat sink is brought to it from the side with the dark side and the readings of the pressure gauge are observed for 1-2 minutes.
Then turn the heat sink with a shiny surface to the lamp located at the same distance from the heat sink, and during the same time monitor the reading of the pressure gauge. They make a conclusion.
In the second series of experiments, the incandescence of the lamp (or the distance to the emitter) is reduced and the change in the pressure gauge readings is again observed under the same conditions. They make a conclusion.
Option 2. See Fig. 2.99; 2.101.
Question. In which case a change in the readings of a liquid manometer
Is it faster if the heat transfer and heat sink face each other with shiny surfaces or if they face each other with black surfaces?



Rice. 123 Fig. 2.101 Fig. 2.99