New ribosomes are formed in the cell. Ribosomes - structure, chemical composition, functions. Free ribosomes, polyribosomes, their connection with other structural components of the cell. Briefly about the structure of the cell
Ribosome (from “RNA” and soma - body) is a cellular non-membrane organelle that carries out translation (reading the mRNA code and synthesizing polypeptides).
Eukaryotic ribosomes are located on the membranes of the endoplasmic reticulum (granular ER) and in the cytoplasm. Ribosomes attached to membranes synthesize protein “for export,” and free ribosomes synthesize protein for the needs of the cell itself. There are 2 main types of ribosomes - prokaryotic and eukaryotic. Mitochondria and chloroplasts also contain ribosomes, which are similar to the ribosomes of prokaryotes.
The ribosome consists of two subunits - large and small. In prokaryotic cells they are designated 50S and 30S subunits, in eukaryotic cells - 60S and 40S. (S is a coefficient that characterizes the sedimentation rate of the subunit during ultracentrifugation). Subunits of eukaryotic ribosomes are formed by self-assembly in the nucleolus and enter the cytoplasm through the pores of the nucleus.
Ribosomes in eukaryotic cells consist of four strands of RNA (three rRNA molecules in the large subunit and one rRNA molecule in the small one) and approximately 80 different proteins, i.e. they represent a complex complex of molecules held together by weak, non-covalent bonds. (Ribosomes in prokaryotic cells consist of three strands of RNA; two strands of rRNA are in the large subunit and one rRNA is in the small subunit). The process of translation (protein biosynthesis) begins with the assembly of an active ribosome. This process is called translation initiation. Assembly occurs in a strictly ordered manner, which is ensured by the functional centers of ribosomes. All centers are located on the contacting surfaces of both ribosomal subunits. Each ribosome works like a large biochemical machine, or more precisely, like a superenzyme, which, firstly, correctly orients the participants (mRNA and tRNA) of the process relative to each other, and secondly, catalyzes reactions between amino acids.
Active sites of ribosomes:
1) mRNA binding center (M-center);
2) peptidyl center (P-center). The initiating tRNA binds to this center at the beginning of the translation process; at subsequent stages of translation, tRNA moves from the A-center to the P-center, holding the synthesized part of the peptide chain;
3) amino acid center (A-center) – the site of binding of the mRNA codon with the anticodon of the tRNA carrying the next amino acid.
4) peptidyl transferase center (PTP center): it catalyzes the binding reaction of amino acids. In this case, another peptide bond is formed, and the growing peptide is lengthened by one amino acid.

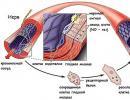
Scheme of protein synthesis on ribosomes of the granular endoplasmic reticulum.
(Figure from the book Cell Biology, Vol.II)

Schematic representation of a polyribosome. Protein synthesis begins with the binding of a small subunit at the location AUG-codon in a messenger RNA molecule (figure from the book Cell Biology, Vol.II).
Endoplasmic reticulum
Endoplasmic reticulum (syn. endoplasmic reticulum) – organelle of a eukaryotic cell. In cells of different types and under different functional states, this component of the cell may look different, but in all cases it is a labyrinthine extended closed membrane structure, built from communicating tube-like cavities and sacs called cisterns. Outside the membranes of the endoplasmic reticulum there is cytosol (hyaloplasm, the main substance of the cytoplasm), and the lumen of the endoplasmic reticulum is a closed space (compartment) communicating through vesicles (transport vesicles) with the Golgi complex and the environment external to the cell. The endoplasmic reticulum is divided into two functionally distinct structures: the granular (rough) endoplasmic reticulum and the smooth (agranular) endoplasmic reticulum.
The granular endoplasmic reticulum, in protein-secreting cells, is represented by a system of numerous flat membrane cisterns with ribosomes on the outer surface. The membrane complex of the granular endoplasmic reticulum is associated with the outer membrane of the nuclear envelope and the perinuclear (perinuclear) cistern.
In the granular endoplasmic reticulum, the synthesis of proteins and lipids for all cell membranes occurs, lysosome enzymes are synthesized, and the synthesis of secreted proteins is also carried out, i.e. intended for exocytosis. (The remaining proteins are synthesized in the cytoplasm on ribosomes not associated with ES membranes.) In the lumen of the granular ES, the protein is surrounded by a membrane, and the resulting vesicles are separated (budding off) from the ribosome-free regions of the ES, which deliver the contents to another organelle - the Golgi complex - by fusion with its membrane.
That part of the ES, on the membranes of which there are no ribosomes, is called the smooth endoplasmic reticulum. The smooth endoplasmic reticulum does not contain flattened cisterns, but is a system of anastomosing membrane channels
ovs, bubbles and tubes. The smooth network is a continuation of the granular network, but does not contain ribophorins - glycoprotein receptors with which the large subunit of ribosomes connects and is therefore not associated with ribosomes.
The functions of the smooth endoplasmic reticulum are diverse and depend on the cell type. The smooth endoplasmic reticulum is involved in the metabolism of steroids, such as sex hormones. Controlled calcium channels and energy-dependent calcium pumps are localized in its membranes. The cisterns of the smooth endoplasmic reticulum are specialized for the accumulation of Ca 2+ in them by constant pumping of Ca 2+ from the cytosol. Similar Ca 2+ depots exist in skeletal and cardiac muscles, neurons, eggs, endocrine cells, etc. Various signals (for example, hormones, neurotransmitters, growth factors) influence cell activity by changing the concentration of the intracellular messenger - Ca 2+. In the smooth endoplasmic reticulum of liver cells, the neutralization of harmful substances (for example, acetaldehyde formed from alcohol), the metabolic transformation of drugs, the formation of most of the cell's lipids and their accumulation occur, for example, in fatty degeneration. The ES cavity contains many different component molecules. Among them are great importance chaperone proteins.

Chaperones(English letters - an elderly lady accompanying a young girl at balls) - a family of specialized intracellular proteins that ensure rapid and correct folding of newly synthesized protein molecules. Binding with chaperones prevents aggregation with other proteins and thereby creates conditions for the formation of the secondary and tertiary structure of the growing peptide. Chaperones belong to three protein families, the so-called heat shock proteins ( hsp 60, hsp 70, hsp90). The synthesis of these proteins is activated under many stresses, in particular during heat shock (hence the nameh eart shook protein – heat shock protein, and the number indicates it molecular weight in kilodaltons). These chaperones prevent denaturation of proteins at high temperatures and other extreme factors. By binding to abnormal proteins, they restore their normal conformation and thereby increase the survival of the organism during a sharp deterioration in the physicochemical parameters of the environment.
Often several ribosomes are associated with one mRNA molecule; this structure is called polyribosome. The synthesis of ribosomes in eukaryotes occurs in a special intranuclear structure - the nucleolus.
Scheme of ribosome synthesis in eukaryotic cells.
1. Synthesis of mRNA for ribosomal proteins by RNA polymerase II. 2. Export of mRNA from the nucleus. 3. Recognition of mRNA by the ribosome and 4. synthesis of ribosomal proteins. 5. Synthesis of rRNA precursor (45S - precursor) by RNA polymerase I. 6. Synthesis of 5S rRNA by RNA polymerase III. 7. Assembly of a large ribonucleoprotein particle, including a 45S precursor, ribosomal proteins imported from the cytoplasm, as well as special nucleolar proteins and RNAs that take part in the maturation of ribosomal subparticles. 8. Attachment of 5S rRNA, cutting of the precursor and separation of the small ribosomal subunit. 9. Maturation of the large subparticle, release of nucleolar proteins and RNA. 10. Release of ribosomal subparticles from the nucleus. 11. Involving them in the broadcast.
Ribosomes are a nucleoprotein, in which the RNA/protein ratio is 1:1 in higher animals and 60-65:35-40 in bacteria. Ribosomal RNA makes up about 70% of the total RNA in a cell. Eukaryotic ribosomes include four rRNA molecules, of which 18S, 5.8S and 28S rRNA are synthesized in the nucleolus by RNA polymerase I as a single precursor (45S), which is then modified and cut. 5S rRNA is synthesized by RNA polymerase III in another part of the genome and does not require additional modifications. Almost all rRNA is in the form of a magnesium salt, which is necessary for maintaining structure; When magnesium ions are removed, the ribosome undergoes dissociation into subunits.
Translation mechanism
Translation is the synthesis of a protein by a ribosome based on information recorded in messenger RNA (mRNA). The mRNA binds to the small subunit of the ribosome when the 3" end of the 16S ribosomal RNA recognizes the complementary Shine-Dalgarno sequence located at the 5" end of the mRNA (in prokaryotes), as well as the positioning of the start codon (usually AUG) of the mRNA on the small subunit . Association of the small and large subunits occurs with the binding of formylmethionyl-tRNA (fMET-tRNA) and the participation of initiation factors (IF1, IF2 and IF3 in prokaryotes; their analogues and additional factors are involved in translation initiation in eukaryotic ribosomes). Thus, anticodon recognition (in tRNA) occurs on the small subunit.
After association, fMET-tRNA ends up in the P (peptidyl-) center of the ribosome. The next tRNA, carrying an amino acid at the 3" end and complementary to the second codon on the mRNA, binds with the help of the EF-Tu factor at the A (aminoacyl) center of the ribosome. Then, on the large subunit, in the peptidyl transferase center of the ribosome, a peptide bond is formed between formylmethionine (bound to tRNA located in the P-center) and the amino acid located in the A-center. Regarding the details of the mechanism of catalysis of the formation peptide bond In the peptidyl transferase center, consensus has not yet been reached. On this moment There are several hypotheses for the mechanism of catalysis by the ribosome: 1. optimal positioning of substrates (induced fit), 2. exclusion from the active center of water that can interrupt the formation of the peptide chain through hydrolysis, 3. participation of rRNA nucleotides (such as A2450 and A2451) in proton transfer, 4 - participation of the 2"-hydroxyl group of the 3"-terminal nucleotide of tRNA (A76) in proton transfer; as well as combinations of these mechanisms.
After the formation of a peptide bond, the polypeptide becomes associated with the tRNA located in the A-center. The next step is the movement of deacylated tRNA from P- to E (exit-) center, and peptidyl-tRNA from A- to P-center. This process is called translocation and occurs with the help of the EF-G factor. tRNA, complementary to the next codon of the mRNA, binds to the A-center of the ribosome, which leads to the repetition of the described steps. Stop codons (UGA, UAG and UAA) signal the end of translation. The termination of the polypeptide chain and the dissociation of subunits (in preparation for the binding of the next mRNA and the synthesis of the corresponding protein) occurs with the participation of factors (RF1, RF2, RF3, RRF in prokaryotes).
Links
External links
The website of one of the leading scientists in the study of the structure of ribosomes contains a large number of illustrations, including animated ones (English)
Wikimedia Foundation. 2010.
See what “Ribosomes” are in other dictionaries:
Modern encyclopedia
Intracellular particles consisting of ribosomal RNA and proteins. By binding to an mRNA molecule, it is translated (protein biosynthesis). Several ribosomes can bind to one mRNA molecule, forming a polyribosome (polysome). Ribosomes... ... Big encyclopedic Dictionary
Ribosomes- RIBOSOMES, intracellular particles consisting of ribosomal RNA and proteins. By binding to a messenger RNA (mRNA) molecule, it is translated (protein biosynthesis). Several ribosomes usually bind to one mRNA molecule, forming a polyribosome... ... Illustrated Encyclopedic Dictionary
Intracellular organelles that carry out protein synthesis. They consist of protein and three types of RNA, connected into a complex by hydrogen and hydrophobic bonds. Constructed from 2 subunits. They differ in sedimentation constant and localization. Bacter. R. doesn’t... ... Dictionary of microbiology
ribosomes- - cell organelles, consisting of RNA and proteins, take part in the biosynthesis of proteins (see translation) ... Brief dictionary biochemical terms
Intracellular particles consisting of ribosomal RNA and proteins. By binding to an mRNA molecule, it is translated (protein biosynthesis). Several ribosomes can bind to one mRNA molecule, forming a polyribosome (polysome). Ribosomes... ... encyclopedic Dictionary
Intracellular particles that carry out protein biosynthesis; R. are found in the cells of all living organisms without exception: bacteria, plants, and animals; each cell contains thousands or tens of thousands of R. The form of R. is close to ... ... Great Soviet Encyclopedia
Intracellular particles consisting of ribosomal RNA and proteins. By binding to an mRNA molecule, it is translated (protein biosynthesis). Several mRNA molecules can bind to one molecule. R., forming a polyribosome (polysome). R. are present in... ... Natural science. encyclopedic Dictionary
- (gr. soma body) intracellular particles consisting of protein and ribonucleic acid and freely lying in the cytoplasm or attached to intracellular membranes; R. serve as a site for protein biosynthesis. New dictionary foreign words. by EdwART,… … Dictionary of foreign words of the Russian language
ribosomes- ribos ohms, ohms, units. h. with oma, s... Russian spelling dictionary
Books
- Molecular biology. Ribosomes and protein biosynthesis. Textbook, Spirin Alexander Sergeevich. Educational edition, written by a leading expert in the field, addresses the structural and functional aspects protein biosynthesis. The book covers part general course molecular...
What does this organelle look like? It looks like a telephone with a receiver. (Fig. 6) The ribosome of eukaryotes and prokaryotes consists of two parts, one of which is larger, the other is smaller. But these two components do not come together when she is in a calm state. This occurs only when the cell's ribosome directly begins to perform its functions. The ribosome also contains messenger RNA and transfer RNA. These substances are necessary in order to record on them information about the proteins needed by the cell. The ribosome does not have its own membrane. Its subunits (as its two halves are called) are not protected by anything.
Figure 6. Appearance ribosomes.
The large subparticle, in turn, consists of:
- · one molecule of ribosomal RNA, which is highly polymeric;
- · one RNA molecule, which is low-polymer;
- · a certain number of protein molecules, usually about three dozen.
As for the smaller subparticle, it’s a little simpler. (Fig. 7) It includes:
- · high-polymer RNA molecule;
- · several dozen protein molecules, usually about 40 (the molecules are varied in structure and shape).

Figure 7. Smaller ribosomal subunit.
A high-polymer RNA molecule is necessary in order to combine all the proteins present into one integral ribonucleoprotein component of the cell.
Functions of the ribosome
What functions does this organelle perform in the cell? What the ribosome is responsible for is protein synthesis. It occurs on the basis of information that is recorded on the so-called messenger RNA (ribonucleic acid). The ribosome unites its two subunits only during protein synthesis, a process called translation. (Fig. 8) During this procedure, the synthesized polypeptide chain is located between two ribosomal subunits.

Figure 8. Translation process.
In the process of performing its main function, that is, during protein synthesis, the ribosome also performs a number of additional ones:
- · Ligament, as well as retention of all components of the so-called protein synthesizing system. It is customary to call this function informational or matrix. The ribosome distributes these functions between its two subparticles, each of which performs its own specific task in this process.
- · Ribosomes perform a catalytic function, which consists in the formation of a special peptide bond (amide bond, which occurs both during the formation of proteins and during the formation of peptides). This also includes the hydrolysis of GTP (the substrate for RNA synthesis). The large subunit of the ribosome is responsible for performing this function. It is in it that there are special areas in which the process of peptide bond synthesis occurs, as well as the center necessary for the hydrolysis of GTP. In addition, it is the large subunit of the ribosome that, during protein biosynthesis, holds on itself a chain that gradually grows.
- · The ribosome performs the functions of mechanical movement of substrates, which include mRNA and tRNA. In other words, they are responsible for translocation.
Figure 9. Protein synthesis.
How are proteins formed? (Fig. 9, 10, 11) Protein biosynthesis occurs in several stages. The first of these is the activation of amino acids. There are twenty of them in total; when combining them using different methods, you can get billions of different proteins. During this stage, aminoalc-tRNA is formed from amino acids.

Figure 10. Protein synthesis (photo).
This procedure is impossible without the participation of ATP (adenosine triphosphoric acid). Also, magnesium cations are needed to carry out this process. The second stage is the initiation of the polypeptide chain, or the process of combining two subunits of the ribosome and supplying it with the necessary amino acids. Magnesium ions and GTP (guanosine triphosphate) also take part in this process. The third stage is called elongation. This is the direct synthesis of the polypeptide chain. Occurs by broadcast method. Termination - the next stage - is the process of disintegration of the ribosome into individual subunits and the gradual cessation of synthesis of the polypeptide chain. Next comes the last stage - the fifth - this is processing. At this stage, a simple chain of amino acids is formed complex structures, which are already ready-made proteins. Specific enzymes and cofactors are involved in this process.

Figure 11. Protein synthesis (scheme).
Since the ribosome is responsible for the synthesis of proteins, let's take a closer look at their structure. It can be primary, secondary, tertiary and quaternary. The primary structure of a protein is a specific sequence in which the amino acids that form a given organic compound are located. The secondary structure of the protein consists of alpha helices and beta folds formed from polypeptide chains. The tertiary structure of a protein involves a specific combination of alpha helices and beta sheets. The quaternary structure consists in the formation of a single macromolecular formation. (Fig. 12) That is, combinations of alpha helices and beta structures form globules or fibrils. Based on this principle, two types of proteins can be distinguished: fibrillar and globular.
The first include such as actin and myosin, from which muscles are formed. Examples of the latter include hemoglobin, immunoglobulin and others. Fibrillar proteins resemble a thread or fiber. Globular ones are more like a ball of intertwined alpha helices and beta folds. What is denaturation? Everyone has probably heard this word.

Figure 12. Quaternary structure of a protein.
ribosome cell protein genetic
Denaturation is the process of destruction of the protein structure - first quaternary, then tertiary, and then secondary. In some cases, the primary structure of the protein is also eliminated. This process may occur due to the impact on this organic matter high temperature. Thus, protein denaturation can be observed when boiling chicken eggs. In most cases, this process is irreversible. So, at a temperature above forty-two degrees, denaturation of hemoglobin begins, so severe hyperthermia is life-threatening. Denaturation of proteins to individual nucleic acids can be observed during the digestion process, when, with the help of enzymes, the body breaks down complex organic compounds into simpler ones.
RIBOSOME (from “ribonucleic acid” and Greek “soma” - body), an organelle that synthesizes proteins. Present in the cells of all organisms, both eukaryotes and prokaryotes. It is a spherical particle with a diameter of approx. 20 nm, consisting of two subparticles that can be separated and reunited. The structural framework of the ribosome is formed by ribosomal RNA (rRNA) molecules and associated proteins. In eukaryotic cells, ribosomes are formed in the nucleolus, where r-RNA is synthesized on DNA, to which proteins are then attached. Subparticles of the ribosome leave the nucleus into the cytoplasm, and here the formation of full-fledged ribosomes is completed. In the cytoplasm, ribosomes are free in the cytoplasmic matrix (hyaloplasm) or attached to the outer membranes of the nucleus and the endoplasmic reticulum. Free ribosomes synthesize proteins for the internal needs of the cell. Ribosomes on membranes form complexes - polyribosomes, which synthesize proteins that enter the Golgi apparatus through the endoplasmic reticulum and are then secreted by the cell. The number of ribosomes in a cell depends on the intensity of protein biosynthesis - there are more of them in the cells of actively growing tissues (plant meristems, embryos, etc.). Chloroplasts and mitochondria have their own small ribosomes; they provide these organelles with autonomous (nucleus-independent) protein biosynthesis (see Translation).
Diagram of the structure of a ribosome sitting on the membrane of the endoplasmic reticulum:
1 - small subunit;
2 - mRNA;
3 - aminoacyl - tRNA;
4 - amino acid;
5 - large subunit;
6 - membrane of the endoplasmic reticulum;
7 - synthesized polypeptide chain.
Each ribosome consists of two subparticles - large and small. Ribosomes consist of approximately equal (by mass) amounts of RNA and protein (i.e., they are ribonucleoprotein particles). The RNA they contain, called ribosomal RNA (rRNA), is synthesized in the nucleolus. Together, both form a complex three-dimensional structure that has the ability to self-assemble.
During protein synthesis on ribosomes, the amino acids from which the polypeptide chain is built are added one after another to the growing chain. The ribosome serves as a binding site for molecules involved in synthesis, i.e., a place where these molecules can take a very specific position in relation to each other. The synthesis involves: messenger RNA (mRNA), which carries genetic instructions from the cell nucleus, transport RNA (tRNA), which delivers the required amino acids to the ribosome, a growing polypeptide chain, as well as a number of factors responsible for the initiation, elongation and termination of the chain.
In eukaryotic cells, two populations of ribosomes are clearly visible - free ribosomes and ribosomes attached to the endoplasmic reticulum. The structure of both is identical, but some of the ribosomes are connected to the endoplasmic reticulum through the proteins that they synthesize. Such proteins are usually secreted. An example of a protein synthesized by free ribosomes is hemoglobin, which is formed in young red blood cells.
During protein synthesis, the ribosome moves along the thread-like mRNA molecule. The process is more efficient when not just one ribosome moves along the mRNA, but many ribosomes at the same time, resembling beads on a string in this case. Such chains of ribosomes are called polyribosomes or polysomes. On the endoplasmic reticulum, polysomes are found in the form of characteristic curls.
Ribosomal protein synthesis is a multistep process. The first stage (initiation) begins with the attachment of messenger RNA (mRNA) to the small ribosomal subunit, which is not associated with the large subunit. It is characteristic that a dissociated ribosome is required to begin the process. To the resulting so-called a large ribosomal subunit is attached to the initiation complex. Specialists participate in the initiation stage. start codon (see Genetic code), initiator transfer RNA (tRNA) and specific. proteins (so-called initiation factors). Having passed the initiation stage, the ribosome moves on to the sequence. reading the codons of mRNA in the direction from the 5" to the 3" end, which is accompanied by the synthesis of the polypeptide chain of the protein encoded by this mRNA (for more information about the mechanism of polypeptide synthesis, see article Translation). In this process, the ribosome functions as a cyclic molecule. car. The working cycle of the ribosome during elongation consists of three cycles: 1) codon-dependent binding of aminoacyl-tRNA (supplies amino acids to the ribosome), 2) transpeptidation - transfer of the C-terminus of the growing peptide to aminoacyl-tRNA, i.e. elongation of the protein chain under construction by one link, 3) translocation-movement of the matrix (mRNA) and peptidyl-tRNA relative to the ribosome and the transition of the ribosome to its original state, when it can perceive the trace. aminoacyl-tRNA. When the ribosome reaches the special stop codon of the mRNA, polypeptide synthesis stops. With the participation of specific proteins (so-called termination factors) synthesized. the polypeptide is released from the ribosome. After termination, the ribosome can repeat the entire cycle with another strand of mRNA or another coding sequence of the same strand.

Scheme of synthesis of a polypeptide chain by a polyribosome: I-beginning of synthesis, II-end of synthesis; a-mRNA, b-ribosome, b-large ribosomal subunit, g-small ribosomal subunit.
In cells with intense protein secretion and developed endoplasmic. reticulum means. part of the cytoplasmic ribosome is attached to its membrane on the surface facing the cytoplasm. These ribosomes synthesize polypeptides, which are directly transported across the membrane for further secretion. The synthesis of polypeptides for intracellular needs occurs mainly. on free (not membrane bound) ribosomes of the cytoplasm. In this case, translating ribosomes are not evenly dispersed in the cytoplasm, but are collected in groups. Such ribosome aggregates are structures where the mRNA is associated with many ribosomes that are in the process of translation; these structures are called polyribosome or polysome.
With intensive protein synthesis, the distance between ribosomes along the mRNA chain in a polyribosome may. extremely short, i.e. ribosomes are located almost close to each other. The ribosomes included in polyribosomes work independently and each of them synthesizes a complete polypeptide chain.
The ribosome is the very worker who transforms general plan into life, making the corresponding proteins based on DNA patterns.
In a bacterial cell, ribosomes make up up to 30% of its dry mass: there are approximately 10 4 ribosomes per bacterial cell. In eukaryotic cells (cells of all organisms, with the exception of bacteria and blue-green algae) refers. the content of ribosomes is smaller, and their number varies greatly depending on the protein-synthesizing activity of the corresponding tissue or individual cell.
In eukaryotic in a cell, all cytoplasmic ribosomes (both membrane-bound and free) are formed in the nucleolus; they are considered to be inactive there. Eukaryotic. the cell also has special ribosomes in mitochondria (in animals and plants) and chloroplasts (in plants). The ribosomes of these organelles differ from the cytoplasmic ones in size and certain functions. Holy you. They are formed directly in these organelles.
There are two main ones. type of ribosomes. Prokaryotic everyone. organisms (bacteria and blue-green algae) are characterized by the so-called. 70S ribosomes, characterized by coefficient. (constant) sedimentation approx. 70 Svedberg units, or 70S (based on the sedimentation coefficient, other types of ribosomes are also distinguished, as well as subparticles and biopolymers that make up ribosomes). They say m is 2.5 · 10 6, linear dimensions 20-25 nm. According to chemistry composition is ribonucleoproteins; they consist only of rRNA and protein (the ratio of these components is 2:1). Ribosomal RNA is present in ribosomes. arr. in the form of Mg-salt (apparently, partially also in the form of Ca-salt); magnesium in ribosomes up to 2% of dry weight. In addition, in different quantity (up to 2.5%) amine cations such as spermine H 2 N(CH 2) 3 NH(CH 2) 4 NH(CH 2) 3 NH 2 and spermidine H 2 N(CH 2) 3 NH may also be present (CH 2) 4 NH 2 etc.
Apparently, rRNA determines the basic structural and functional. The properties of ribosomes, in particular, ensure the integrity of ribosomal subunits, determine their shape and a number of structural features. Specific spaces. The rRNA structure determines the localization of all ribosomal proteins and plays a leading role in the organization of functions. ribosome centers.
Ribosomal protein synthesis is a multistep process. The first stage (initiation) begins with the attachment of messenger RNA (mRNA) to the small ribosomal subunit, which is not associated with the large subunit. It is characteristic that a dissociated ribosome is required to begin the process. To the resulting so-called a large ribosomal subunit is attached to the initiation complex. Specialists participate in the initiation stage. initiation codon (see Genetic code), initiation transfer RNA (tRNA) and specific. proteins (so-called initiation factors). Having passed the initiation stage, the ribosome moves on to the sequence. reading the codons of mRNA in the direction from the 5" to the 3" end, which is accompanied by the synthesis of the polypeptide chain of the protein encoded by this mRNA (for more information about the mechanism of polypeptide synthesis, see article Translation). In this process, the ribosome functions as a cyclic molecule. car. The working cycle of the ribosome during elongation consists of three cycles: 1) codon-dependent binding of aminoacyl-tRNA (supplies amino acids to the ribosome), 2) transpeptidation - transfer of the C-terminus of the growing peptide to aminoacyl-tRNA, i.e. elongation of the protein chain under construction by one link, 3) translocation-movement of the matrix (mRNA) and peptidyl-tRNA relative to the ribosome and the transition of the ribosome to its original state, when it can perceive the trace. aminoacyl-tRNA. When the ribosome reaches the special