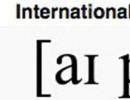Chemically pure iron properties and applications. Chemical properties of iron. Foods rich in iron
Iron was known in prehistoric times, but it was widely used much later, since it is extremely rare in nature in the free state, and its production from ores became possible only at a certain level of technological development. Probably, for the first time, a person became acquainted with meteorite Iron, as evidenced by its names in the languages of ancient peoples: the ancient Egyptian "beni-pet" means "heavenly iron"; the ancient Greek sideros is associated with the Latin sidus (genus case sideris) - a star, a celestial body. In the Hittite texts of the 14th century BC. e. Iron is mentioned as a metal that fell from the sky. In the Romance languages, the root of the name given by the Romans has been preserved (for example, French fer, Italian ferro).
The method of obtaining Iron from ores was invented in the western part of Asia in the 2nd millennium BC. e.; after that, the use of Iron spread in Babylon, Egypt, Greece; The Bronze Age was replaced by the Iron Age. Homer (in the 23rd song of the Iliad) tells that Achilles awarded the winner of the discus throwing competition with an iron cry discus. In Europe and Ancient Russia for many centuries, iron was obtained by the cheese-making process. Iron ore was reduced with charcoal in a furnace built in a pit; air was pumped into the hearth with furs, the reduction product - kritsu was separated from the slag by hammer blows and various products were forged from it. As the methods of blowing were improved and the height of the hearth increased, the temperature of the process increased and part of the iron became carburized, that is, cast iron was obtained; this relatively fragile product was considered a waste product. Hence the name of cast iron "chushka", "pig iron" - English. pig iron. Later it was noticed that when not iron ore, but cast iron is loaded into the hearth, low-carbon iron bloom is also obtained, and such a two-stage process turned out to be more profitable than raw-dough. In the 12th-13th centuries, the screaming method was already widespread.
In the 14th century, cast iron began to be smelted not only as a semi-finished product for further processing, but also as a material for casting various products. The reconstruction of the hearth into a shaft furnace (“domnitsa”), and then into a blast furnace, also dates back to the same time. In the middle of the 18th century, the crucible process of obtaining steel began to be used in Europe, which was known in Syria in the early period of the Middle Ages, but later was forgotten. With this method, steel was obtained by melting a metal charge in small vessels (crucibles) from a highly refractory mass. In the last quarter of the 18th century, the puddling process of converting cast iron into iron began to develop on the hearth of a fiery reverberatory furnace. The industrial revolution of the 18th and early 19th centuries, the invention of the steam engine, the construction of railways, large bridges, and the steam fleet created an enormous demand for iron and its alloys. However, all existing methods of iron production could not meet the needs of the market. Mass production of steel began only in the middle of the 19th century, when the Bessemer, Thomas and open-hearth processes were developed. In the 20th century, the electric steelmaking process arose and became widespread, giving high quality steel.
Distribution of iron in nature. In terms of content in the lithosphere (4.65% by weight), iron ranks second among metals (aluminum is in first place). It migrates vigorously in the earth's crust, forming about 300 minerals (oxides, sulfides, silicates, carbonates, titanates, phosphates, etc.). Iron takes an active part in magmatic, hydrothermal and hypergene processes, which are associated with the formation of various types of its deposits. Iron is a metal of the earth's depths, it accumulates in the early stages of magma crystallization, in ultrabasic (9.85%) and basic (8.56%) rocks (it is only 2.7% in granites). In the biosphere, iron accumulates in many marine and continental sediments, forming sedimentary ores.
An important role in the geochemistry of iron is played by redox reactions - the transition of 2-valent iron to 3-valent and vice versa. In the biosphere, in the presence of organic substances, Fe 3+ is reduced to Fe 2+ and easily migrates, and when it encounters atmospheric oxygen, Fe 2+ is oxidized, forming accumulations of trivalent iron hydroxides. Widespread compounds of 3-valent Iron are red, yellow, brown. This determines the color of many sedimentary rocks and their name - "red-colored formation" (red and brown loams and clays, yellow sands, etc.).
Physical properties of iron. The importance of iron in modern technology is determined not only by its wide distribution in nature, but also by a combination of very valuable properties. It is plastic, easily forged both in a cold and heated state, can be rolled, stamped and drawn. The ability to dissolve carbon and other elements is the basis for obtaining a variety of iron alloys.
Iron can exist in the form of two crystal lattices: α- and γ-body-centered cubic (bcc) and face-centered cubic (fcc). Below 910°C, α-Fe with a bcc lattice is stable (a = 2.86645Å at 20°C). Between 910°C and 1400°C, the γ-modification with the fcc lattice is stable (a = 3.64Å). Above 1400°C, the δ-Fe bcc lattice (a = 2.94Å) is again formed, which is stable up to the melting point (1539°C). α-Fe is ferromagnetic up to 769 °C (Curie point). Modifications γ-Fe and δ-Fe are paramagnetic.
Polymorphic transformations of iron and steel during heating and cooling were discovered in 1868 by D.K. Chernov. Carbon forms interstitial solid solutions with Iron, in which C atoms having a small atomic radius (0.77 Å) are located at the interstices of the metal crystal lattice, which consists of larger atoms (Fe atomic radius 1.26 Å). A solid solution of carbon in γ-Fe is called austenite, and in α-Fe it is called ferrite. A saturated solid solution of carbon in γ-Fe contains 2.0% C by mass at 1130 °C; α-Fe dissolves only 0.02-0.04% C at 723 °C, and less than 0.01% at room temperature. Therefore, when austenite is quenched, martensite is formed - a supersaturated solid solution of carbon in α-Fe, which is very hard and brittle. The combination of quenching with tempering (heating to relatively low temperatures to reduce internal stresses) makes it possible to give the steel the required combination of hardness and ductility.
The physical properties of Iron depend on its purity. In industrial iron materials Iron is usually accompanied by impurities of carbon, nitrogen, oxygen, hydrogen, sulfur, and phosphorus. Even at very low concentrations, these impurities greatly change the properties of the metal. So, sulfur causes the so-called red brittleness, phosphorus (even 10 -2% P) - cold brittleness; carbon and nitrogen reduce plasticity, and hydrogen increases the brittleness of Iron (the so-called hydrogen brittleness). Reducing the content of impurities to 10 -7 - 10 -9% leads to significant changes in the properties of the metal, in particular to an increase in ductility.
The following are the physical properties of Iron, referring mainly to a metal with a total impurity content of less than 0.01% by mass:
Atomic radius 1.26Å
Ionic radii Fe 2+ 0.80Å, Fe 3+ 0.67Å
Density (20°C) 7.874 g/cm3
t bale about 3200°С
Temperature coefficient of linear expansion (20°C) 11.7 10 -6
Thermal conductivity (25°C) 74.04 W/(m K)
The heat capacity of Iron depends on its structure and changes in a complex way with temperature; average specific heat capacity (0-1000°C) 640.57 j/(kg K) .
Electrical resistivity (20°C) 9.7 10 -8 ohm m
Temperature coefficient of electrical resistance (0-100°C) 6.51 10 -3
Young's modulus 190-210 10 3 MN / m 2 (19-21 10 3 kgf / mm 2)
Temperature coefficient of Young's modulus 4 10 -6
Shear modulus 84.0 10 3 MN/m 2
Short-term tensile strength 170-210 MN/m2
Relative elongation 45-55%
Brinell hardness 350-450 MN/m2
Yield strength 100 MN/m2
Impact strength 300 MN/m2
Chemical properties of iron. The configuration of the outer electron shell of the atom is 3d 6 4s 2 . Iron exhibits a variable valency (the most stable compounds are 2- and 3-valent Iron). With oxygen, Iron forms oxide (II) FeO, oxide (III) Fe 2 O 3 and oxide (II,III) Fe 3 O 4 (compound of FeO with Fe 2 O 3 having a spinel structure). In humid air at ordinary temperatures, iron becomes covered with loose rust (Fe 2 O 3 nH 2 O). Due to its porosity, rust does not prevent the access of oxygen and moisture to the metal and therefore does not protect it from further oxidation. As a result of various types of corrosion, millions of tons of Iron are lost every year. When iron is heated in dry air above 200 °C, it is covered with a very thin oxide film, which protects the metal from corrosion at ordinary temperatures; this is the basis of the technical method of protecting Iron - bluing. When heated in water vapor, iron is oxidized to form Fe 3 O 4 (below 570 °C) or FeO (above 570 °C) and release hydrogen.
Hydroxide Fe (OH) 2 is formed as a white precipitate by the action of caustic alkalis or ammonia on aqueous solutions of Fe 2+ salts in an atmosphere of hydrogen or nitrogen. When in contact with air, Fe(OH) 2 first turns green, then turns black, and finally quickly turns into red-brown Fe(OH) 3 hydroxide. FeO oxide exhibits basic properties. Oxide Fe 2 O 3 is amphoteric and has a mildly acidic function; reacting with more basic oxides (for example, with MgO), it forms ferrites - compounds of the Fe 2 O 3 nMeO type, which have ferromagnetic properties and are widely used in radio electronics. Acidic properties are also expressed in 6-valent Iron, which exists in the form of ferrates, for example K 2 FeO 4 , salts of iron acid not isolated in the free state.
Iron easily reacts with halogens and hydrogen halides, giving salts, such as chlorides FeCl 2 and FeCl 3 . When iron is heated with sulfur, FeS and FeS 2 sulfides are formed. Iron carbides - Fe 3 C (cementite) and Fe 2 C (e-carbide) - precipitate from solid solutions of carbon in iron upon cooling. Fe 3 C is also released from solutions of carbon in liquid Iron at high concentrations of C. Nitrogen, like carbon, gives interstitial solid solutions with Iron; nitrides Fe 4 N and Fe 2 N are isolated from them. With hydrogen, iron gives only slightly stable hydrides, the composition of which has not been precisely established. When heated, iron reacts vigorously with silicon and phosphorus to form silicides (eg Fe 3 Si and phosphides (eg Fe 3 P).
Iron compounds with many elements (O, S and others), which form a crystalline structure, have a variable composition (for example, the sulfur content in monosulfide can vary from 50 to 53.3 at.%). This is due to defects in the crystal structure. For example, in iron oxide (II), some of the Fe 2+ ions at the lattice sites are replaced by Fe 3+ ions; to maintain electrical neutrality, some lattice sites belonging to Fe 2+ ions remain empty.
The normal electrode potential of Iron in aqueous solutions of its salts for the reaction Fe = Fe 2+ + 2e is -0.44 V, and for the reaction Fe = Fe 3+ + 3e is -0.036 V. Thus, in the series of activities, iron is to the left of hydrogen. It readily dissolves in dilute acids with the release of H 2 and the formation of Fe 2+ ions. The interaction of iron with nitric acid is peculiar. Concentrated HNO 3 (density 1.45 g/cm 3) passivates Iron due to the formation of a protective oxide film on its surface; more dilute HNO 3 dissolves Iron with the formation of Fe 2+ or Fe 3+ ions, being reduced to NH 3 or N 2 and N 2 O. Solutions of salts of 2-valent Iron in air are unstable - Fe 2+ gradually oxidizes to Fe 3+. Aqueous solutions of iron salts are acidic due to hydrolysis. The addition of thiocyanate ions SCN- to solutions of Fe 3+ salts gives a bright blood-red color due to the appearance of Fe(SCN) 3, which makes it possible to reveal the presence of 1 part of Fe 3+ in about 10 6 parts of water. Iron is characterized by the formation of complex compounds.
Getting Iron. Pure iron is obtained in relatively small quantities by the electrolysis of aqueous solutions of its salts or by the reduction of its oxides with hydrogen. The production of sufficiently pure iron is gradually increasing by means of its direct reduction from ore concentrates with hydrogen, natural gas, or coal at relatively low temperatures.
The use of iron. Iron is the most important metal of modern technology. In its pure form, due to its low strength, iron is practically not used, although steel or cast iron products are often called "iron" in everyday life. The bulk of iron is used in the form of alloys with very different compositions and properties. Iron alloys account for approximately 95% of all metal products. Carbon-rich alloys (over 2% by weight) - cast iron, are smelted in blast furnaces from iron-rich ores. Steel of various grades (carbon content less than 2% by mass) is smelted from cast iron in open-hearth and electric furnaces and converters by oxidizing (burning out) excess carbon, removing harmful impurities (mainly S, P, O) and adding alloying elements. High-alloy steels (with a high content of nickel, chromium, tungsten and other elements) are smelted in electric arc and induction furnaces. New processes such as vacuum and electroslag remelting, plasma and electron-beam melting, and others are used for the production of steels and iron alloys for particularly important purposes. Methods are being developed for smelting steel in continuously operating units that ensure high quality of the metal and automation of the process.
Iron-based materials are created that can withstand the effects of high and low temperatures, vacuum and high pressures, aggressive media, high alternating voltages, nuclear radiation, etc. The production of iron and its alloys is constantly growing.
Iron as an art material has been used since ancient times in Egypt, Mesopotamia, and India. Since the Middle Ages, numerous highly artistic iron products have been preserved in European countries (England, France, Italy, Russia and others) - forged fences, door hinges, wall brackets, weather vanes, chest fittings, lights. Forged through products from rods and products from perforated sheet iron (often with a mica lining) are distinguished by planar shapes, a clear linear-graphic silhouette and are effectively visible against a light-air background. In the 20th century, iron is used for the manufacture of lattices, fences, openwork interior partitions, candlesticks, and monuments.
Iron in the body. Iron is present in the organisms of all animals and in plants (about 0.02% on average); it is necessary mainly for oxygen exchange and oxidative processes. There are organisms (the so-called concentrators) capable of accumulating it in large quantities (for example, iron bacteria - up to 17-20% of Iron). Almost all of the iron in animal and plant organisms is associated with proteins. Iron deficiency causes growth retardation and plant chlorosis associated with reduced chlorophyll production. An excess of iron also has a harmful effect on the development of plants, causing, for example, sterility of rice flowers and chlorosis. In alkaline soils, iron compounds that are inaccessible to plant roots are formed, and plants do not receive it in sufficient quantities; in acidic soils, iron passes into soluble compounds in excess. With a deficiency or excess of assimilable iron compounds in soils, plant diseases can be observed in large areas.
Iron enters the body of animals and humans with food (liver, meat, eggs, legumes, bread, cereals, spinach, and beets are the richest in iron). Normally, a person receives 60-110 mg of Iron with the diet, which significantly exceeds his daily requirement. The absorption of iron ingested with food occurs in the upper part of the small intestines, from where it enters the blood in a protein-bound form and is carried with the blood to various organs and tissues, where it is deposited in the form of an iron-protein complex - ferritin. The main depot of iron in the body is the liver and spleen. Due to ferritin, all the iron-containing compounds of the body are synthesized: the respiratory pigment hemoglobin is synthesized in the bone marrow, myoglobin is synthesized in the muscles, and cytochromes and other iron-containing enzymes are synthesized in various tissues. Iron is excreted from the body mainly through the wall of the large intestine (in humans, about 6-10 mg per day) and to a small extent by the kidneys. The body's need for Iron varies with age and physical condition. For 1 kg of weight, children need - 0.6, adults - 0.1 and pregnant women - 0.3 mg of Iron per day. In animals, the need for Iron is approximately (per 1 kg of dry matter of the diet): for dairy cows - at least 50 mg, for young animals - 30-50 mg; for piglets - up to 200 mg, for pregnant pigs - 60 mg.
Details Category: Views: 9555 IRON, Fe, chemical element, atomic weight 55.84, serial number 26; located in the VIII group of the periodic system in the same row with cobalt and nickel, melting point - 1529 ° C, boiling point - 2450 ° C; in the solid state has a bluish-silver color. In free form, iron is found only in meteorites, which, however, contain admixtures of Ni, P, C, and other elements. In nature, iron compounds are widely distributed throughout (soil, minerals, animal hemoglobin, plant chlorophyll), Ch. arr. in the form of oxides, hydrates of oxides and sulfur compounds, as well as iron carbonate, of which most iron ores are composed.
IRON, Fe, chemical element, atomic weight 55.84, serial number 26; located in the VIII group of the periodic system in the same row with cobalt and nickel, melting point - 1529 ° C, boiling point - 2450 ° C; in the solid state has a bluish-silver color. In free form, iron is found only in meteorites, which, however, contain admixtures of Ni, P, C, and other elements. In nature, iron compounds are widely distributed throughout (soil, minerals, animal hemoglobin, plant chlorophyll), Ch. arr. in the form of oxides, hydrates of oxides and sulfur compounds, as well as iron carbonate, of which most iron ores are composed.
Chemically pure iron is obtained by heating oxalic iron, and at 440 ° C, at first, an opaque powder of ferrous oxide is obtained, which has the ability to ignite in air (the so-called pyrophoric iron); with the subsequent reduction of this oxide, the resulting powder acquires a gray color and loses its pyrophoric properties, turning into metallic iron. During the reduction of ferrous oxide at 700° C., iron precipitates in the form of small crystals, which are then fused in vacuum. Another way to obtain chemically pure iron is the electrolysis of a solution of iron salts, such as FeSO 4 or FeCl 3 mixed with MgSO 4 , CaCl 2 or NH 4 Cl (at temperatures above 100°C). However, at the same time, iron occludes a significant amount of electrolytic hydrogen, as a result of which it acquires hardness. When calcined to 700 ° C, hydrogen is released, and iron becomes soft and is cut with a knife, like lead (hardness on the Mohs scale is 4.5). Very pure iron can be obtained aluminothermally from pure iron oxide. (see Aluminothermy). Well-formed iron crystals are rare. Octahedral crystals sometimes form in the cavities of large pieces of cast iron. A characteristic property of iron is its softening, malleability and ductility at a temperature much lower than the melting point. When iron is exposed to strong nitric acid (which does not contain lower nitrogen oxides), iron is covered with a coating of oxides and becomes insoluble in nitric acid.
Iron compounds
Easily combining with oxygen, iron forms several oxides: FeO - ferrous oxide, Fe 2 O 3 - iron oxide, FeO 3 - ferric anhydride and FeO 4 - anhydride of ironic acid. In addition, iron also forms an oxide of the mixed type Fe 3 O 4 - ferrous oxide, the so-called. iron scale. In dry air, however, iron does not oxidize; rust is an aqueous iron oxide formed with the participation of air moisture and CO 2 . Ferrous oxide FeO corresponds to hydrate Fe (OH) 2 and a number of salts of divalent iron, capable of being oxidized into salts of iron oxide, Fe 2 O 3, in which iron manifests itself as a trivalent element; in air, iron oxide hydrate, which has strong reducing properties, is easily oxidized, turning into iron oxide hydrate. Ferrous oxide hydrate is slightly soluble in water, and this solution has a clearly alkaline reaction, indicating the basic character of ferrous iron. Iron oxide is found in nature (see. Iron minium), while artificially m. obtained in the form of a red powder by calcining iron powder and by burning sulfur pyrites to obtain sulfur dioxide. Anhydrous iron oxide, Fe 2 O 3, m. obtained in two modifications, and the transition from one of them to another occurs when heated and is accompanied by a significant release of heat (self-heating). With strong calcination, Fe 2 O 3 releases oxygen and passes into magnetic oxide, Fe 3 O 4. Under the action of alkalis on solutions of ferric iron salts, a precipitate of hydrate Fe 4 O 9 H 6 (2Fe 2 O 3 3H 2 O) precipitates; when it is boiled with water, Fe 2 O 3 ·H 2 O hydrate is formed, which is difficult to dissolve in acids. Iron forms compounds with various metalloids: with C, P, S, with halides, as well as with metals, for example, with Mn, Cr, W, Cu, etc.
Iron salts are divided into ferrous - ferrous iron (ferro-salt) and oxide - ferric iron (ferri-salt).
ferrous salts . ferric chloride, FeCl 2 , obtained by the action of dry chlorine on iron, in the form of colorless leaves; when iron is dissolved in HCl, ferric chloride is obtained in the form of FeCl 2 4H 2 O hydrate and is used in the form of aqueous or alcoholic solutions in medicine. Iron iodide, FeJ 2 , is obtained from iron and iodine under water in the form of green leaves and is used in medicine (Sirupus ferri jodati); with further action of iodine, FeJ 3 (Liquor ferri sesquijodati) is formed.
ferrous sulfate, ferrous sulfate, FeSO 4 7H 2 O (green crystals) is formed in nature as a result of the oxidation of pyrite and sulfur pyrites; this salt is also formed as a by-product in the production of alum; when weathered or when heated to 300 ° C, it turns into a white anhydrous salt - FeSO 4; also forms hydrates with 5, 4, 3, 2 and 1 water particles; easily soluble in cold water (in hot water up to 300%); the solution is acidic due to hydrolysis; oxidizes in air, especially easily in the presence of another oxidizing substance, for example, oxalic acid salts, which FeSO 4 involves in a coupled oxidation reaction, discolors KMnO 4; the process proceeds according to the following equation:
2KMnO 4 + 10FeSO 4 + 8H 2 SO 4 \u003d 2MnSO 4 + K 2 SO 4 + 5Fe 2 (SO 4) 2 + 8H 2 O.
For this purpose, however, the more permanent double salt of Mohr (NH 4) 2 Fe (SO 4) 2 6H 2 O is used. Iron sulfate is used in gas analysis to determine nitric oxide absorbed by a solution of FeSO 4 with the formation of dark -brown color of the (FeNO)SO 4 complex, as well as for the production of ink (with tannic acids), as a stain for dyeing, for binding malodorous gases (H 2 S, NH 3) in latrines, etc.
Iron ferrous salts are used in photography due to their ability to reduce silver compounds in a latent image imprinted on a photographic plate.
iron carbonate, FeCO 3 , occurs naturally as siderite or iron spar; obtained by precipitation of aqueous solutions of iron ferrous salts with carbonates, iron carbonate easily loses CO 2 and is oxidized in air to Fe 2 O 3.
Iron bicarbonate, H 2 Fe (CO 3) 2, soluble in water and occurs naturally in ferruginous sources, from which, oxidized, it is released on the surface of the earth in the form of iron oxide hydrate, Fe (OH) 3, turning into brown iron ore.
Phosphate iron, Fe 3 (PO 4) 2 8H 2 O, white precipitate; occurs in nature slightly colored, due to the oxidation of iron, in a blue color, in the form of vivianite.
Iron oxide salts . Ferric chloride, FeCl 3 (Fe 2 Cl 6), is obtained by the action of excess chlorine on iron in the form of hexagonal red plates; ferric chloride dissolves in air; crystallizes from water in the form of FeCl 3 6H 2 O (yellow crystals); solutions are acidic; during dialysis, it is gradually hydrolyzed almost to the end with the formation of a colloidal solution of Fe (OH) 3 hydrate. FeCl 3 dissolves in alcohol and in a mixture of alcohol and ether, when heated, FeCl 3 6H 2 O decomposes into HCl and Fe 2 O 3; used as a dressing and as a hemostatic agent (Liquor ferri sesquichlorati).
Sulphate oxide iron, Fe 2 (SO 4) 3 , yellowish in anhydrous state, highly hydrolyzed in solution; when the solution is heated, basic salts precipitate; ferrous alum, MFe(SO 4) 2 12H 2 O, M - monovalent alkali metal; ammonium alum crystallize best of all, NH 4 Fe (SO 4) 2 12H 2 O.
The oxide FeO 3 is an anhydride of iron acid, as well as the hydrate of this oxide H 2 FeO 4 - ferric acid- in a free state not m. obtained in view of their extreme fragility; but in alkaline solutions there may be salts of iron acid, ferrates (for example, K 2 FeO 4), which are formed by heating iron powder with nitrate or KClO 3. Also known sparingly soluble barium salt of iron acid BaFeO 4 ; thus, ferric acid is in some respects very similar to sulfuric and chromic acids. In 1926, the Kyiv chemist Goralevich described compounds of octavalent iron oxide - supraferrous anhydride FeO 4 obtained by fusing Fe 2 O 3 with saltpeter or Bertolet salt in the form of potassium salt of ironic acid K 2 FeO 5; FeO 4 is a gaseous substance that does not form ironic acid H 2 FeO 5 with water, which, however, can. isolated in the free state by decomposition of salt K 2 FeO 5 with acids. The barium salt BaFeO 5 7H 2 O, as well as calcium and strontium salts, were obtained by Goralevich in the form of non-decomposing white crystals that release water only at 250-300 ° C and turn green at the same time.
Iron gives compounds: with nitrogen - nitrous iron(nitride) Fe 2 N when iron powder is heated in a jet of NH 3 , with carbon - Fe 3 C carbide when iron is saturated with coal in an electric furnace. In addition, a number of compounds of iron with carbon monoxide have been studied - iron carbonyls, for example, pentacarbonyl Fe(CO) 5 - slightly colored liquid with about 102.9 ° C (at 749 mm, specific gravity 1.4937), then an orange solid Fe 2 (CO) 9, insoluble in ether and chloroform, with specific gravity 2.085.
Of great importance are iron cyanide compounds. In addition to simple cyanides Fe (CN) 2 and Fe (CN) 3, iron forms a number of complex compounds with cyanide salts, such as salts of ferric acid H 4 Fe (CN) 6, and salts of ferric acid H 3 Fe (CN) 6, for example, red blood salt, which, in turn, enter into metabolic decomposition reactions with salts of ferrous and oxide iron, forming blue-colored compounds - Prussian blue and turnbull blue. When replacing one CN group with monovalent groups (NO, NO 2, NH 3, SO 3, CO) in the salts of ferruginous acid H 4 Fe (CN) 6 , Prusso salts are formed, for example, sodium nitroprusside (nitroferric-cyanogen sodium) Na 2 2H 2 O, obtained by the action of fuming HNO 3 on K 4 Fe (CN) 6, followed by neutralization with soda, in the form of ruby-red crystals, separated by crystallization from the saltpeter formed simultaneously; the corresponding nitroferric-cyanotic acid H 2 also crystallizes as dark red crystals. Sodium nitroprusside is used as a sensitive reagent for hydrogen sulfide and metal sulfides, with which it gives a blood-red, then turning into blue, color. Under the action of copper sulphate on sodium nitroprusside, a pale green precipitate, insoluble in water and alcohol, is formed, which is used to test essential oils.
Analytically, iron is detected by the action on its salts, in an alkaline solution, of the yellow blood salt. Salts of ferric iron form a blue precipitate of Prussian blue. Salts of ferrous iron form a blue precipitate of turnbull blue when exposed to red blood salt. With ammonium thiocyanate NH 4 CNS, ferric iron salts form water-soluble, blood-red colored rhodan iron Fe(CNS) 3 ; with tannin, iron oxide salts form ink. The copper salts of ferric-cyanotic acid are also distinguished by intense coloration, which are used (uvachrome method) in color photography. Of the iron compounds used in medicine, in addition to the mentioned iron halides, the following are important: metallic iron (F. hydrogenio reductum), iron citrate (F. Citricum - 20% Fe), malic iron extract (Extractum ferri pomatum), iron albuminate ( Liquor ferri albuminatum), ferratin is a protein compound with 6% iron; ferratose - a solution of ferratin, carniferrin - a compound of iron with nuclein (30% Fe); ferratogen from yeast nuclein (1% Fe), hematogen - 70% solution of hemoglobin in glycerol, hemol - hemoglobin reduced by zinc dust.
Physical properties of iron
The numerical data available in the literature characterizing the various physical properties of iron fluctuate due to the difficulty of obtaining iron in a chemically pure state. Therefore, the most reliable are the data obtained for electrolytic iron, in which the total content of impurities (C, Si, Mn, S, P) does not exceed 0.01-0.03%. The data below in most cases refer to such hardware. For it, the melting point is 1528°C ± 3°C (Ruer and Klesper, 1914), and the boiling point is ≈ 2450°C. In the solid state, iron exists in four different modifications - α, β, γ and δ, for which the following temperature limits are fairly accurately established:

The transition of iron from one modification to another is detected on the cooling and heating curves by critical points, for which the following designations are accepted:

 These critical points are shown in Fig. 1 with schematic heating and cooling curves. The existence of δ-, γ- and α-Fe modifications is currently considered indisputable, while the independent existence of β-Fe is disputed due to the insufficiently sharp difference between its properties and those of α-Fe. All modifications of iron crystallize in the form of a cube, and α, β and δ have a spatial lattice of a centered cube, and γ-Fe - a cube with centered faces. The most distinct crystallographic characteristics of iron modifications are obtained from X-ray spectra, as shown in Fig. 2 (Westgreen, 1929).
These critical points are shown in Fig. 1 with schematic heating and cooling curves. The existence of δ-, γ- and α-Fe modifications is currently considered indisputable, while the independent existence of β-Fe is disputed due to the insufficiently sharp difference between its properties and those of α-Fe. All modifications of iron crystallize in the form of a cube, and α, β and δ have a spatial lattice of a centered cube, and γ-Fe - a cube with centered faces. The most distinct crystallographic characteristics of iron modifications are obtained from X-ray spectra, as shown in Fig. 2 (Westgreen, 1929).  It follows from the presented X-ray diffraction patterns that for α-, β-, and δ-Fe the lines of the X-ray spectrum are the same; they correspond to the lattice of a centered cube with parameters 2.87, 2.90 and 2.93 Ȧ, and for γ-Fe the spectrum corresponds to the lattice of a cube with centered faces and parameters 3.63-3.68 A.
It follows from the presented X-ray diffraction patterns that for α-, β-, and δ-Fe the lines of the X-ray spectrum are the same; they correspond to the lattice of a centered cube with parameters 2.87, 2.90 and 2.93 Ȧ, and for γ-Fe the spectrum corresponds to the lattice of a cube with centered faces and parameters 3.63-3.68 A.
The specific gravity of iron ranges from 7.855 to 7.864 (Cross and Gill, 1927). When heated, the specific gravity of iron decreases due to thermal expansion, for which the coefficients increase with temperature, as shown in Table. 1 (Driesen, 1914).

The decrease in the expansion coefficients in the ranges of 20–800°C, 20–900°C, 700–800°C, and 800–900°C is explained by anomalies in the expansion upon passing through the critical points A C2 and A C3 . This transition is accompanied by contraction, especially pronounced at point A C3 as shown by the contraction and expansion curves in FIG. 3. The melting of iron is accompanied by its expansion by 4.4% (Gonda and Enda, 1926). The heat capacity of iron is quite significant in comparison with other metals and is expressed for different temperature ranges from 0.11 to 0.20 Cal, as shown in Table. 2 (Obergoffer and Grosse, 1927) and the curve constructed from them (Fig. 4).

 In the given data, the transformations A 2 , A 3 , A 4 and the melting of iron are found so clearly that thermal effects are easily calculated for them: A 3 ... + 6.765 Cal, A 4 ... + 2.531 Cal, iron melting ... - 64.38 Cal (according to S. Umino, 1926, - 69.20 Cal).
In the given data, the transformations A 2 , A 3 , A 4 and the melting of iron are found so clearly that thermal effects are easily calculated for them: A 3 ... + 6.765 Cal, A 4 ... + 2.531 Cal, iron melting ... - 64.38 Cal (according to S. Umino, 1926, - 69.20 Cal).
Iron is characterized by approximately 6-7 times lower thermal conductivity than silver, and 2 times lower than aluminum; namely, the thermal conductivity of iron is at 0°C - 0.2070, at 100°C - 0.1567, at 200°C - 0.1357 and at 275°C - 0.1120 Cal/cm·s·°С. The most characteristic properties of iron are magnetic, expressed by a number of magnetic constants obtained during a complete cycle of iron magnetization. These constants for electrolytic iron are expressed by the following values in gauss (Gumlich, 1909 and 1918):

When passing through point A c2, the ferromagnetic properties of iron almost disappear and can be. detected only with very precise magnetic measurements. In practice, β-, γ-, and δ-modifications are considered non-magnetic. The electrical conductivity for iron at 20°C is R -1 mo m/mm 2 (where R is the electrical resistance of iron, equal to 0.099 Ω mm 2 /m). The temperature coefficient of electrical resistance a0-100 ° x10 5 ranges from 560 to 660, where
![]()
 Cold working (rolling, forging, broaching, stamping) has a very noticeable effect on the physical properties of iron. So, their % change during cold rolling is expressed by the following figures (Gerens, 1911): coercive voltage + 323%, magnetic hysteresis + 222%, electrical resistance + 2%, specific gravity - 1%, magnetic permeability - 65%. The latter circumstance makes understandable those significant fluctuations in physical properties that are observed by different researchers: the influence of impurities is often accompanied by the influence of cold mechanical treatment.
Cold working (rolling, forging, broaching, stamping) has a very noticeable effect on the physical properties of iron. So, their % change during cold rolling is expressed by the following figures (Gerens, 1911): coercive voltage + 323%, magnetic hysteresis + 222%, electrical resistance + 2%, specific gravity - 1%, magnetic permeability - 65%. The latter circumstance makes understandable those significant fluctuations in physical properties that are observed by different researchers: the influence of impurities is often accompanied by the influence of cold mechanical treatment.
Very little is known about the mechanical properties of pure iron. Electrolytic iron fused in a void found: tensile strength 25 kg / mm 2, elongation - 60%, cross-sectional compression - 85%, Brinell hardness - from 60 to 70.
The structure of iron depends on the content of impurities in it (even in small quantities) and the pre-treatment of the material. The microstructure of iron, like other pure metals, consists of more or less large grains (crystallites), which are called ferrite here.


The sizes and sharpness of their outlines depend ch. arr. on the iron cooling rate: the lower the latter, the more developed the grains and the sharper their contours. From the surface, the grains are most often unequally colored due to unequal crystallography, their orientation, and the unequal etching action of reagents in different directions in the crystal. It is not uncommon for grains to be elongated in one direction as a result of mechanical processing. If the processing took place at low temperatures, then shear lines (Neumann lines) appear on the surface of the grains as a result of the sliding of individual parts of the crystallites along their cleavage planes. These lines are one of the signs of hardening and those changes in properties that were mentioned above.
Iron in metallurgy
The term iron in modern metallurgy is assigned only to wrought iron, i.e., a low-carbon product obtained in a pasty state at a temperature not sufficient to melt iron, but so high that its individual particles are well welded to each other, giving after forging a homogeneous soft product , not accepting hardening. Iron (in the indicated sense of the word) is obtained: 1) directly from the ore in a paste-like state by a cheese-blowing process; 2) in the same way, but at a lower temperature, insufficient for welding iron particles; 3) redistribution of cast iron by the blooming process; 4) redistribution of cast iron by puddling.
1) Cheese blowing process in present. time is used only by uncultured peoples and in such areas where (due to the lack of convenient means of communication) American or European iron, obtained by modern methods, cannot penetrate. The process is carried out in open raw furnaces and furnaces. The raw materials for it are iron ore (usually brown iron ore) and charcoal. Coal is poured into the hearth in that half of it where the blast is supplied, while the ore is in a heap, from the opposite side. Carbon monoxide formed in a thick layer of burning coal passes through the entire thickness of the ore and, having a high temperature, reduces iron. Ore recovery is carried out gradually - from the surface of individual pieces to the core. Starting at the top of the heap, it accelerates as the ore moves into an area of higher temperature; in this case, iron oxide passes first into magnetic oxide, then into oxide, and, finally, metallic iron appears on the surface of the ore pieces. At the same time, earthy impurities of the ore (waste rock) are combined with iron oxide that has not yet been reduced and form a low-melting ferrous slag, which melts through the cracks of the metal shell, which forms, as it were, a shell in each piece of ore. Being heated to a white-hot heat, these shells are welded to each other, forming a spongy mass of iron at the bottom of the hearth - a kritsu, penetrated by slag. To separate from the latter, the kritsa taken out of the hearth is cut into several parts, each of which is forged, welded, after cooling in the same hearth into strips or directly into products (household items, weapons). In India, the cheese-making process is still carried out in cheese-making furnaces, which differ from furnaces only in a slightly higher height - about 1.5 m. The walls of the furnaces are made of clay mass (not brick) and serve only one smelting. The blast is fed into the furnace through one tuyere by bellows driven by feet or hands. A certain amount of charcoal (“idle head”) is loaded into an empty furnace, and then alternately, in separate layers, ore and coal, with the amount of the first gradually increasing until it reaches a certain relationship to coal; the weight of the whole ore filled is determined by the desired weight of the bloom, which, generally speaking, is negligible. The recovery process is the same as in the forge; iron is also not completely restored, and the resulting bloom contains a lot of ferruginous slag. Kritsu is extracted by breaking the oven and cut into pieces, 2-3 kg in weight. Each of them is heated in a forge and processed under a hammer; the result is an excellent soft iron, which, among other things, is the material for the manufacture of Indian steel "woots" (damask steel). Its composition is as follows (in%):
The negligible content of elements - iron impurities - or their complete absence is explained by the purity of the ore, the incomplete reduction of iron and the low temperature in the furnace. The consumption of charcoal due to the small size of furnaces and furnaces and the frequency of their action is very high. In Finland, Sweden and the Urals, iron was smelted in the Husgavel cheese-blast furnace, in which it was possible to control the process of reduction and saturation of iron with carbon; coal consumption in it - up to 1.1 per unit of iron, the output of which reached 90% of its content in the ore.
2) In the future, it is necessary to expect the development of iron production directly from ore, not by using a raw blast process, but by reducing iron at a temperature insufficient for the formation of slag and even for sintering waste ore (1000 ° C). The advantages of such a process are the possibility of using low-grade fuels, the elimination of flux and the heat consumption for slag melting.
3) The production of wrought iron by the redistribution of cast iron by the blooming process is carried out in the blooming furnaces of Ch. arr. in Sweden (we have - in the Urals). For redistribution, special cast iron is smelted, the so-called. Lancashire, giving the least waste. It contains: 0.3-0.45% Si, 0.5-0.6% Mn, 0.02 P,<0,01% S. Такой чугун в изломе кажется белым или половинчатым. Горючим в кричных горнах может служить только древесный уголь.
The process is being followed. arr.: the hearth, freed from the cry, but with the ripe slag of the end of the process remaining on the bottom board, is filled with coal, ch. arr. pine, on which cast iron heated by combustion products is laid in the amount of 165-175 kg (for 3/8 m 2 of the cross section of the hearth there are 100 kg of cast iron cages). By turning the valve in the air duct, the blast is directed through the pipes located in the under-roof space of the hearth, and is heated here to a temperature of 150-200 ° C, thus accelerating. melting iron. The melting pig iron is constantly supported (with the help of crowbars) on the coal above the tuyeres. During such work, the entire mass of cast iron is subjected to the oxidative action of atmospheric oxygen and carbon dioxide, passing through the combustion zone in the form of droplets. Their large surface contributes to the rapid oxidation of iron and its impurities - silicon, manganese and carbon. Depending on the content of these impurities, cast iron loses them to a greater or lesser extent before it collects at the bottom of the hearth. Since low-silicon and low-manganese cast iron is reworked in the Swedish forge, then, passing the tuyere horizon, it loses all its Si and Mn (the oxides of which form the main slag with ferrous oxide) and a significant part of the carbon. Cast iron melting lasts 20-25 minutes. At the end of this process, cold blast is put into the forge. The metal that has settled to the bottom of the hearth begins to react with ripe slags located there, containing a large excess (compared to the amount of silica) of iron oxides - Fe 3 O 4 and FeO, oxidizing carbon with the release of carbon monoxide, which boils the entire metal. When the metal thickens (from the loss of carbon) and "sits down as a commodity", the latter is lifted with crowbars above the lances, hot blast is again started and the "commodity" is melted.
During the secondary melting, the metal is oxidized by the oxygen of both the blast and the slags that are melted out of it. At the bottom of the forge, after the first rise, metal falls, soft enough to collect kritsu from some of the most ripe parts of it. But before, when using silicon grades of cast iron, it was necessary to resort to a second and even third rise in goods, which, of course, reduced the productivity of the furnace, increased fuel consumption and iron waste. The results of the work were influenced by the distance of the lances from the bottom board (the depth of the hearth) and the slope of the lances: the steeper the lance is set and the smaller the depth of the hearth, the greater the effect of the oxidizing atmosphere on the metal. The more gentle slope of the lances, as well as the greater depth of the hearth, reduces the direct action of the blast oxygen, thus giving a greater role to the action of the slag on the iron impurities; oxidation by them is slower, but without iron fumes. Under any given conditions, the most advantageous position of the lances relative to the bottom board is determined by experience; in a modern swedish forge, the eye of the lance is set at a distance of 220 mm from the bottom board, and the inclination of the tuyeres varies within close limits - from 11 to 12°.
The crack obtained at the bottom of the hearth contains, in contrast to the raw blow, very little mechanically entrained slag; as for the chemical impurities of iron, then Si, Mn and C can be. are completely removed (the negligible content of Si and Mn indicated by the analyzes is part of the mechanical impurity - slag), and sulfur is only partially oxidized by blast during melting. At the same time, phosphorus is also oxidized, going into the slag in the form of a phosphoric iron salt, but the latter is then reduced by carbon, and the final metal can contain even relatively more phosphorus (from iron fumes) than the original cast iron. That is why, in order to obtain first-class metal for export in Sweden, exclusively pure pig iron in relation to P is taken into the redistribution. The finished kritsa taken out of the forge is cut into three parts (each 50-55 kg) and pressed under a hammer, giving the appearance of a parallelepiped.
The duration of the redistribution process in the Swedish bloomery is from 65 to 80 minutes; from 2.5 to 3.5 tons of compressed pieces “for fire” are obtained per day, with a consumption of charcoal of only 0.32-0.40 per unit of finished material and its output from 89 to 93.5% of the cast iron specified in the redistribution. Most recently, successful experiments have been made in Sweden in the conversion of liquid iron taken from blast furnaces, and in accelerating the boiling process by stirring the metal with a mechanical rake; while waste decreased to 7%, and coal consumption - to 0.25.
The following data (in%) give the concept of the chemical composition of Swedish and South Ural iron:
Of all the types of iron obtained by industrial methods, Swedish bloomery is the closest to chemically pure and is used instead of the latter in laboratory practice and research work. It differs from raw iron in its uniformity, and from the softest open-hearth metal (cast iron) in the absence of manganese; it is characterized by the highest degree of weldability, ductility and malleability. Swedish bloomery iron exhibits negligible tensile strength of only about 30 kg/mm 2 , with an elongation of 40% and a reduction in cross section of 75%. At present, the annual production of bloomery iron in Sweden has fallen to 50,000 tons, since after the war of 1914-18. the scope of industrial applications for this iron has been greatly reduced. The largest amount of it is used for the manufacture (in England of the main arr. and in Germany) of the highest grades of tool and special steels; in Sweden itself, it is used to make special wire (“flower”), horseshoe nails, well forged in a cold state, chains and strip blanks for welded pipes. For the last two purposes, the properties of bloomery iron are especially important: reliable weldability, and for pipes, moreover, the highest resistance to rusting.
4) The development of iron production by the blooming process entailed the destruction of forests; after the latter were taken under the protection of a law in various countries, which limited their felling to an annual increase, Sweden, and then Russia - wooded countries abounding in high quality ores - became the main suppliers of iron on the international market throughout the 18th century. In 1784, the Englishman Cort invented puddling - the process of redistribution of cast iron on the hearth of a fiery furnace, in the furnace of which coal was burned. After Cort's death, Rogers and Gall introduced significant improvements in the design of the puddling furnace, which contributed to the rapid spread of puddling in all industrial countries and completely changed the nature and extent of their iron production during the first half of the 19th century. By this process, they obtained the mass of metal that was needed for the construction of iron ships, railways, locomotives, steam boilers and cars.
The fuel for puddling is long-flame bituminous coal, but where it is not available, we had to resort to brown coal, and here in the Urals - to firewood. Pine wood gives a longer flame than hard coal; it heats well, but the moisture content in the wood should not exceed 12%. Subsequently, the Siemens regenerative oven was used for puddling in the Urals. Finally, in the USA and in our country (in the Volga and Kama basins) puddling furnaces operated on oil sprayed directly into the working space of the furnace.
For speed of redistribution and reduction of fuel consumption, it is desirable to have cold puddling cast iron; when smelting it on coke, however, a lot of sulfur is obtained in the product (0.2 and even 0.3%), and with a high content of phosphorus in the ore, phosphorus. For ordinary commercial grades of iron, such pig iron with a low silicon content (less than 1%), called pig iron, was previously smelted in large quantities. Charcoal cast iron, which was reworked in the Urals and in central Russia, did not contain sulfur and gave a product that was also used for the manufacture of roofing iron. At present, puddling is used to produce high-quality metal according to special specifications, and therefore not ordinary pig iron is supplied to puddling furnaces, but high-quality, for example, manganese or "hematite" (low phosphorus), or, conversely, highly phosphorus for the production of nut iron. Below is the content (in%) of the main elements in some grades of cast iron used for puddling:

The puddling furnace, at the end of the previous operation, usually has a normal amount of slag on the bottom to work with the next charge. When processing strongly silicon cast iron, a lot of slag remains in the furnace, and it has to be lowered; on the contrary, white cast iron is left “dry” under the furnace, and work has to be started by throwing in the required amount of slag, which is taken from under the hammer (“ripe”, the most rich in magnetic oxide). An iron charge is thrown onto the slag, heated in a cast iron (250-300 kg in ordinary and 500-600 kg in double furnaces); then a fresh portion of fuel is thrown into the furnace, the grate is cleaned, and full draft is installed in the furnace. Within 25-35 min. cast iron melts, undergoing b. or m. a significant change in its composition. Hard cast iron is oxidized by the oxygen of the flame, and iron, manganese and silicon give a double silicate flowing down on the hearth of the furnace; melting cast iron exposes more and more layers of solid cast iron, which also oxidizes and melts. At the end of the melting period, two liquid layers are formed on the hearth - cast iron and slag, on the contact surface of which carbon is oxidized, albeit to a weak degree, by magnetic iron oxide, as evidenced by bubbles of carbon monoxide released from the bath. Depending on the content of silicon and manganese in cast iron, an unequal amount of them remains in the molten metal: in low-silicon charcoal iron or white - coke melting - silicon in most cases burns out completely during melting; sometimes a certain amount of it remains in the metal (0.3-0.25%), as well as manganese. Phosphorus is also oxidized at this time, turning into a phosphoric iron salt. From a decrease in the weight of the metal during the burnout of these impurities, the % carbon content may even increase, although some of it is undoubtedly burned by the oxygen of the flame and slag covering the first portions of the molten metal.
To accelerate the burnout of the remaining amounts of silicon, manganese and carbon, puddling is resorted to, i.e., mixing cast iron with slag using a club with a right-angled end. If the metal is liquid (grey cast iron, highly carbonaceous), then the mixing does not reach the goal, and the bath is first made thick by throwing cold ripe slag into it, or by reducing the draft, incomplete combustion is set in the furnace, accompanied by a very smoky flame (languishing). After a few minutes, during which continuous stirring is carried out, abundant bubbles of burning carbon monoxide appear on the surface of the bath - a product of the oxidation of cast iron carbon by oxygen of magnetic oxide dissolved in the main ferrous slag. As the process progresses, the oxidation of C intensifies and turns into a violent “boiling” of the entire mass of the metal, which is accompanied by its swelling and such a significant increase in volume that part of the slag overflows through the threshold of the working holes. As C burns out, the melting point of the metal rises, and in order for the boiling to continue, the temperature in the furnace is continuously increased. Boiling completed at a low temperature gives a raw product, i.e., a high-carbon spongy mass of iron, unable to weld; ripe goods “sit down” in a hot oven. The process of oxidation of iron impurities in a puddling furnace begins with the oxygen of the slag, which is an alloy of iron monosilica (Fe 2 SiO 4) with magnetic oxide and iron oxide of variable composition. In English furnaces, the composition of the mixture of oxides is expressed by the formula 5Fe 3 O 4 5 FeO; at the end of boiling, the ratio of oxides in the depleted slag is expressed by the formula Fe 3 O 4 5FeO, i.e., 80% of the entire magnetic oxide of the slag takes part in the oxidation process. Oxidation reactions m. b. are represented by the following thermochemical equations:

As can be seen from these equations, the oxidation of Si, P and Mn is accompanied by the release of heat and, therefore, heats the bath, while the oxidation of C during the reduction of Fe 3 O 4 to FeO absorbs heat and therefore requires a high temperature. This explains the order of removal of iron impurities and the fact that carbon burnout ends sooner in a hot furnace. Fe 3 O 4 is not reduced to metal, because this requires a higher temperature than that at which “boiling” occurs.
The shrunken "goods", in order to become well-welded iron, still need to be steamed: the goods are left for several minutes in the oven and from time to time they are turned over with crowbars, and its lower parts are placed on top; under the combined action of the oxygen of the flame and slag, impregnating the entire mass of iron, carbon at this time continues to burn out. As soon as a certain amount of well-welded metal is obtained, screams begin to roll out of it, avoiding excessive oxidation. In total, from 5 to 10 kritz are rolled as the goods ripen (no more than 50 kg each); The crackers are kept (steamed) at the threshold in the area of the highest temperature and fed under the hammer for compression, which achieves the separation of slag, and giving them the shape of a piece (section from 10x10 to 15x15 cm), convenient for rolling in rolls. To the place of issued shouts, the following ones move forward by moving forward, until the last one. The duration of the process in the production of the highest quality metal (fibrous iron) from ripe (high-carbon) charcoal cast iron in the Urals was as follows: 1) planting cast iron - 5 minutes, 2) melting - 35 minutes, 3) languishing - 25 minutes, 4) puddling (mixing) - 20 min., 5) steaming the goods - 20 min., 6) knurling and steaming the crackers - 40 minutes, 7) issuing the crackers (10-11 pieces) - 20 minutes; total - 165 min. When working on white cast iron, on ordinary commercial iron, the duration of the process was reduced (in Western Europe) to 100 and even 75 minutes.
As for the results of the work, in different metallurgical regions they varied depending on the type of fuel, the quality of cast iron and the grade of iron produced. The Ural stoves, which worked on wood, gave a yield of usable iron per 1 m 3 of firewood from 0.25 to 0.3 tons; oil consumption per unit of iron is 0.33, coal in European furnaces is from 0.75 to 1.1. The daily output of our large stoves (600 kg of cast iron) when working on dried firewood was 4-5 tons; the output of material suitable for the production of roofing iron was 95-93% of the amount of cast iron supplied to the process. In Europe, the daily productivity of ordinary furnaces (cage 250-300 kg) is about 3.5 tons with a loss of 9%, and for high-quality iron - 2.5 tons with a loss of 11%.
In terms of chemical composition and physical properties, puddling iron is a much worse product than blooming iron, on the one hand, and cast open-hearth iron, on the other. The ordinary grades of iron previously produced in Western Europe contained a lot of sulfur and phosphorus, since they were produced from impure coke irons, and both of these harmful impurities only partially pass into slag; the amount of slag in puddling iron is 3-6%, in high-quality metal it does not exceed 2%. The presence of slag greatly reduces the results of mechanical tests of puddling iron. Below are some data in% characterizing puddling iron - ordinary Western European and good Ural:

A valuable property, for the sake of which the production of puddling iron is now supported, is its excellent weldability, which is sometimes of particular importance from the point of view of safety. Railway specifications Societies require the manufacture of puddling iron coupling devices, rods for transfer switches and bolts. Due to its better resistance to the corrosive action of water, puddling iron is also used for the production of water pipes. It is also used to make nuts (phosphorous coarse metal) and high-quality fibrous iron for rivets and chains.
The structure of wrought iron, detected under a microscope even at low magnification, is characterized by the presence of black and light components in the photographic image; the former belong to the slag, and the latter to the iron grains or fibers obtained by drawing the metal.
Trade iron
Metallurgical plants produce iron of two main types for the needs of industry: 1) sheet and 2) high-quality.
Sheet iron is currently rolled up to 3 m wide; with a thickness of 1-3 mm, we call it fine-rolled; from 3 mm and above (usually up to 40 mm) - boiler, tank, ship, depending on the purpose, which corresponds to the composition and mechanical properties of the material. The softest is boiler iron; it usually contains 0.10-0.12% C, 0.4-0.5% Mn, P and S - each not more than 0.05%; its temporary resistance to rupture is not b. more than 41 kg / mm 2 (but not less than 34 kg / mm 2), elongation at break - about 28%. Reservoir iron is made more solid and durable; it contains 0.12-0.15% C; 0.5-0.7% Mn and not more than 0.06% of both P and S; tear resistance 41-49 kg/mm 2 , elongation 25-28%. The length of the sheets of boiler and tank iron is set by order according to the dimensions of the product riveted from the sheets (avoiding unnecessary seams and trimmings), but usually it does not exceed 8 m, as it is limited for thin sheets by their rapid cooling during the rolling process, and for thick sheets - by the weight of the ingot .
Sheet iron less than 1 mm thick is called tinplate; it is used for making tinplate and as a roofing material. For the latter purpose, in the USSR, sheets are rolled with dimensions of 1422x711 mm, weighing 4-5 kg, with a thickness of 0.5-0.625 mm. Roofing iron is produced by factories in packs weighing 82 kg. Abroad, black tin is classified in trade according to special caliber numbers - from 20 to 30 (the normal thickness of German tin is from 0.875 to 0.22 mm, and English - from 1.0 to 0.31 mm). Tin is made from the softest cast iron, containing 0.08-0.10% C, 0.3-0.35% Mn, if it is made from charcoal cast iron (we have it), and 0.4-0.5% Mn, if the starting material is coke pig iron; tear resistance - from 31 to 34 kg / mm 2, elongation - 28-30%. A variety of sheet iron is corrugated (corrugated) iron. It is divided according to the nature of the waves into iron with low and high waves; in the first, the ratio of wave width to depth ranges from 3 to 4, in the second, 1-2. Corrugated iron is made with a thickness of 0.75-2.0 mm and a sheet width of 0.72-0.81 m (with low waves) and 0.4-0.6 m (with high waves). Corrugated iron is used for roofs, walls of light structures, blinds, and with high waves, in addition, it is used for the construction of rafterless ceilings.
Sectional iron is divided into two classes according to the cross-sectional shape: ordinary sectional iron and shaped.
The first class includes round iron (with a diameter of less than 10 mm called wire), square, flat or strip. The latter, in turn, is divided into: the strip itself - from 10 to 200 mm wide and more than 5 mm thick; hoop - the same width, but with a thickness of 5 to 1 mm, indicated by the caliber number (from the 3rd to the 19th normal German and from the 6th to the 20th new English caliber); tire - from 38 to 51 mm wide and up to 22 mm thick; universal - from 200 to 1000 mm wide and at least 6 mm thick (rolled in special rolls - universal). Both tire and hoop iron are produced by factories in slopes, rolled wire - in coils; other grades - in the form of straight (straightened) strips, usually no more than 8 m long (normal - from 4.5 to 6 m), but by special order for concrete structures, strips are cut up to 18 mm long, and sometimes more.
The main types of shaped iron: angular (equilateral and unequal), box-shaped (channel), tee, I-beam (beam), column (square) and zet iron; there are also some other less common types of shaped iron. According to our normal metric assortment, the dimensions of shaped iron are indicated by the profile number (# - the number, see the width of the shelf or the maximum height of the profile). Angular unequal and tee iron have a double number; for example, No. 16/8 means corner with shelves of 16 and 8 cm or tee with a shelf of 16 cm and a tee height of 8 cm. - double tee.
The composition of ordinary weldable sectional iron: 0.12% C, 0.4% Mn, less than 0.05% P and S - each; its tear resistance is 34-40 kg/mm 2 ; but round iron for rivets is made from a softer material of composition: less than 0.10% C, 0.25-0.35% Mn, about 0.03% P and S each. Tensile strength 32-35 kg/mm 2 and elongation 28-32%. Shaped not weldable, but riveted iron (“building steel”) contains: 0.15 - 0.20% C, 0.5% Mn, up to 0.06% P and S - each; its tear resistance is 40-50 kg/mm 2 , elongation is 25-20%. For the production of nuts, iron (Thomas) is made, containing about 0.1% C, but from 0.3 to 0.5% P (the larger the nuts, the more P). Abroad, to meet the needs of special rolling mills, a semi-finished product is circulated in trade - a square billet, usually 50 x 50 mm in cross section.
IRON (Ferrum, Fe) - an element of group VIII of the periodic system of D. I. Mendeleev; is part of the respiratory pigments, including hemoglobin, is involved in the process of binding and transporting oxygen to tissues in the body of animals and humans; stimulates the function of hematopoietic organs; It is used as a medicine for anemic and some other pathological conditions. The radioactive isotope 59 Fe is used as a radioactive tracer in a wedge, laboratory researches. Ordinal number 26, at. weight 55.847.
Four stable isotopes of iron have been found in nature, with mass numbers 54 (5.84%), 56 (91.68%), 57 (2.17%), and 58 (0.31%).
Iron is found everywhere, both on the Earth, especially in its core, and in meteorites. The earth's crust contains 4.2 weight percent, or 1.5 atomic percent iron. The content of iron in stony meteorites averages 23%, and sometimes reaches 90% (such meteorites are called iron meteorites). In the form of complex organic compounds iron is a part of plant and animal organisms.
Zh. is a part of many minerals, which are iron oxides (red iron ore - Fe 2 O 3, magnetic iron ore - FeO-Fe 2 O 3, brown iron ore - 2Fe 2 O 3 -3H 2 O), or carbonates (siderite - FeCO 3), or sulfur compounds (iron pyrite, magnetic pyrite), or, finally, silicates (eg, olivine, etc.). Zh. is found in ground waters and waters of various reservoirs. Zh. is contained in sea water in a concentration of 5 10 -6%.
In the technique of zinc, it is used in the form of alloys with other elements that significantly change its properties. Iron alloys with carbon are of the greatest importance.
Physico-chemical properties of iron and its compounds
Pure Zh. - a brilliant white malleable metal with a grayish tint; t° pl 1539 ± 5°, t° boiling approx. 3200°; beats weight 7.874; possesses, in comparison with other pure metals, the highest ferromagnetic properties, i.e., the ability to acquire the properties of a magnet under the influence of an external magnetic field.
Two crystalline modifications of iron are known: alpha and gamma iron. The first, alpha modification, is stable below 911° and above 1392°, the second, gamma modification, in the temperature range from 911° to 1392°. At temperatures above 769°, alpha iron is non-magnetic, and below 769°, it is magnetic. Non-magnetic alpha iron is sometimes called beta iron, and high temperature alpha iron is sometimes called delta iron. Zh. easily interacts with diluted acids (for example, with hydrochloric, sulfuric, acetic) with the release of hydrogen and the formation of the corresponding ferrous salts of Zh., i.e., Fe (II) salts. Zh.'s interaction with highly diluted nitric acid occurs without hydrogen evolution with the formation of the ferrous nitrate salt of Zh. - Fe (NO 3) 2 and nitrogen ammonium salt - NH 4 NO 3. At interaction Zh. with konts. nitric acid forms an oxide salt Zh., i.e., a salt of Fe (III), - Fe (NO 3) 3, and nitrogen oxides are simultaneously released.
In dry air, iron is covered with a thin (3 nm thick) oxide film (Fe 2 O 3), but does not rust. At high temperatures, in the presence of air, iron is oxidized, forming iron scale - a mixture of oxide (FeO) and oxide (Fe 2 O 3) Zh. In the presence of moisture and air, iron corrodes; it oxidizes with the formation of rust, the edge is a mixture of hydrated iron oxides. To protect the iron from rusting, it is covered with a thin layer of other metals (zinc, nickel, chromium, etc.) or with oil paints and varnishes, or the formation of iron on the surface is achieved. thin film of nitrous oxide - Fe 3 O 4 (bluing of steel).
Zh. belongs to the elements with variable valence, and therefore its compounds are able to take part in redox reactions. Compounds of bi-, tri- and hexavalent iron are known.
The most stable are compounds of bi- and trivalent iron. Oxygen compounds Zh. - oxide (FeO) and oxide (Fe 2 O 3) - have basic properties and form salts with to-tami. The hydrates of these oxides Fe(OH) 2 , Fe(OH) 3 are insoluble in water. Salts of ferrous, i.e. divalent, liquid (FeCl 2, FeSO 4, etc.), called Fe (II) salts or ferrosalts, are colorless in the anhydrous state, and in the presence of crystallization water or in the dissolved state they have a bluish green color;, they dissociate with the formation of Fe 2+ ions. The crystalline hydrate of double ammonium sulphate and divalent J. (NH 4) 2 SO 4 -FeSO 4 -6H 2 O is called Mohr's salt. A sensitive reaction to salts of Fe (II) is the formation of a precipitate of turnbull blue - Fe 3 2 with the K 3 Fe (CN) 6 solution.
Salts of oxide, i.e., trivalent iron or Fe (III), called Fe (III) salts or ferrisols, are yellow-brown or red-brown in color, for example, ferric chloride, which is commercially available in the form of yellow hygroscopic crystal hydrate FeCl 3 -6H 2 O. Double sulfate salts of Fe (III), called iron alum, for example, iron-ammonium alum (NH 4) 2 SO 4 Fe 2 (SO 4) 3 24H 2 O. In the solution of Fe salts (III) dissociate with the formation of Fe 3+ ions. Sensitive reactions to Fe (III) salts are: 1) the formation of a precipitate of Prussian blue Fe 4 3 with a solution of K 4 Fe (CN) 6 and 2) the formation of red rhodan iron Fe (CNS) 3 with the addition of thiocyanate salts (NH 4 CNS or KCNs).
Compounds of hexavalent iron are salts of iron to-you (ferrates K2FeO4, BaFeO4). Corresponding to these salts iron to - that (H2FeO4) and its anhydride are unstable and in a free state are not received. Ferrates are strong oxidizing agents, they are unstable and easily decompose with the release of oxygen.
There are a large number of complex compounds of liquid. For example, when potassium cyanide is added to the salts of ferrous liquid, potassium cyanide first forms a precipitate of cyanide liquid. Fe (CN) 2, which then, with an excess of KCN, dissolves again to form K 4 Fe (CN) 6 [hexacyano- (II) potassium ferrate, potassium ferricyanide, or potassium ferricyanide]. Another example is K 3 Fe (CN) 6 [potassium hexacyano-(III) ferrate, potassium ferricyanide, or potassium ferrocyanide], etc. Ferrocyanide gives the Fe (CN) 4 - ion in solution, and ferricinide gives the Fe ( CN) 6 3- . Zh., contained in these anions, does not give qualitative reactions to iron ions Fe 3+ and Fe 2+. Zh. easily forms complex compounds with many organic acids, as well as with nitrogenous bases. The formation of colored complex compounds of iron with a, alpha1-dipyridyl or with o-phenanthroline underlies very sensitive methods for detecting and quantifying small amounts of iron. Substances such as heme (see Hemoglobin) of biogenic origin are also complex compounds of iron.
With carbon monoxide, iron gives volatile compounds - carbonyls. Carbonyl Zh. Fe (CO) 5 is called pentacarbonyl Zh. and is used to obtain the most pure, free from any impurities Zh. for the purposes of obtaining chemical. catalysts, as well as for some electrical purposes.
Iron in the human body
The body of an adult contains an average of 4-5 g of Fe, of which approx. 70% is in the composition of hemoglobin, (see), 5-10% - in the composition of myoglobin (see), 20-25% in the form of reserve Zh. and no more than 0.1% - in the blood plasma. A nek-swarm quantity Zh. is a part of various organic compounds intracellularly. OK. 1% Zh. is also part of a number of respiratory enzymes (see Respiratory pigments, Respiratory enzymes, Biological oxidation), which catalyze the processes of respiration in cells and tissues.
Zh., found in a blood plasma, is a transport form Zh., a cut is connected with the transferrin protein representing beta-globulins and, possibly, alpha-globulins and albumins. Theoretically, 1.25 micrograms of fat can be associated with 1 mg of protein, i.e., in total, approx. 3 mg Zh. However, in fact, transferrin is saturated with Zh. only by 20-50% (an average of one third). Additional quantity Zh., a cut in certain conditions can contact transferrin, defines the unsaturated iron-binding ability (NZhSS) of blood; total amount Zh., a cut can be connected by transferrin, defines the general iron-binding ability (OZHSS) of blood. In the blood serum, the content of Zh. is determined according to Valkvist (V. Vahlquist) in the modification of Hagberg (V. Hagberg) and E. A. Efimova. The method is based on the fact that iron-protein complexes in blood plasma in an acidic environment dissociate with the release of F. Proteins are precipitated, and in a protein-free filtrate, Fe (III) is converted into Fe (II), which forms a colored soluble complex with o-phenanthroline, the color intensity is horn is proportional to the amount of Zh. in the solution. For determination, 0.3 ml of non-hemolyzed blood serum is taken, the calculation is made according to the calibration curve.
The iron-binding ability of blood serum is determined by A. Schade in the modification of Rath (C. Rath) and Finch (C. Finch). The method is based on the fact that the interaction of beta-globulins and divalent iron produces an orange-red complex. Therefore, when ferrosalts (usually Mohr's salts) are added to the blood serum, the intensity of this color increases, edges sharply stabilize at the saturation point of the protein. By quantity Zh., necessary for saturation of protein, judge NZhSS. This value, summed up with the amount of fluid in the blood serum, reflects OZHSS.
Zh.'s maintenance in a blood plasma is subject to daily fluctuations; it decreases by the second half of the day. Zh.'s concentration in a blood plasma also depends on age: at newborns it is equal to 175 mcg%, at children at the age of 1 year - 73 mcg%; then the concentration of Zh. again increases to 110-115 μg% and does not change significantly until the age of 13. In adults, there are differences in the concentration of Zh. in the blood serum depending on gender: the content of Zh. in men is 120 mcg%, and in women - 80 mcg%. In whole blood, this difference is less pronounced. OZHSS of normal blood serum is 290-380 mcg%. In the urine of a person, 60-100 mcg of F is excreted per day.
Deposition of iron in tissues
Zh., which is deposited in the tissues of the body, can be of exogenous and endogenous origin. Exogenous siderosis is observed in some professions as an occupational hazard, in particular among miners employed in the development of red iron ore, and among electric welders. In these cases, Fe (III) oxides (Fe 2 O 3) are deposited in the lungs, sometimes with the formation of siderotic nodules diagnosed by radiography. Histologically, the nodules are an accumulation of dust containing iron in the lumen of the alveoli, in desquamated alveolar cells, in the interalveolar septa, in the adventitia of the bronchi with development around the connective tissue. In electric welders, the amount of fluid deposited in the lungs is usually small; its particles are predominantly less than 1 micron; massive deposits are observed in miners., the amount to-rogo in both lungs can reach 45 g and make up 39.6% of the weight of the ash remaining after the combustion of the lung. Pure siderosis of the lungs, for example, in electric welders, is not accompanied by pneumosclerosis and disability; miners, however, usually have sidero-silicosis with the development of pneumosclerosis (see).
Exogenous siderosis of the eyeball is observed when iron fragments, shavings, etc. are introduced into the eye; at the same time, metallic fluid passes into bicarbonate, then into hydrate of fluid oxide and is deposited in the processes of the ciliary body, the epithelium of the anterior chamber, the lens capsule, the episcleral tissue, the retina, and the optic nerve, where it can be detected using the appropriate microchem. reactions. Exogenous local siderosis can be observed around iron fragments that have fallen into tissues during household and combat trauma (fragments of grenades, shells, etc.).
The source of endogenous siderosis in the vast majority of cases is hemoglobin with its extra- and intravascular destruction. One of the end products of hemoglobin breakdown is the iron-containing pigment hemosiderin, which is deposited in organs and tissues. Hemosiderin was discovered in 1834 by I. Müller, but the term "hemosiderin" was proposed by A. Neumann only later, in 1888. Hemosiderin is formed by cleavage of heme. It is a polymer of ferritin (see) [Granik (S. Granick)]. Chemically, hemosiderin is an aggregate of Fe(III) hydroxide more or less firmly bound to proteins, mucopolysaccharides, and cell lipids. The formation of hemosiderin occurs in cells of both mesenchymal and epithelial nature. These cells
V. V. Serov and V. S. Paukov proposed to call them sideroblasts. Hemosiderin granules are synthesized in siderosomes of sideroblasts. Microscopically, hemosiderin has the appearance of grains from yellowish to golden brown, located mostly inside the cells, but sometimes extracellularly. Hemosiderin granules contain up to 35% Zh.; hemosiderin never forms crystalline forms.
Due to the fact that the source of hemosiderin in most cases is hemoglobin, focal deposits of the latter can be observed anywhere in the human body where hemorrhage has occurred (see Hemosiderosis). In hemosiderosis, SH-ferritin (active sulfhydryl form), which has vasoparalytic properties, is detected in the places of hemosiderin deposition. Especially large deposits of hemosiderin, arising from ferritin due to a violation of cellular metabolism Zh., are observed with hemochromatosis (see); while in the liver the amount of deposited fat often exceeds 20-30 g. Deposits of fat in hemochromatosis, in addition to the liver, are observed in the pancreas, kidneys, myocardium, organs of the reticuloendothelial system, sometimes the mucous glands of the trachea, in the thyroid gland, muscles and epithelium of the tongue etc.
In addition to deposits of hemosiderin, sometimes there is impregnation (ferruginization) of the elastic skeleton of the lungs, elastic membranes of the vessels of the lung with brown induration, or cerebral vessels in the circumference of the hemorrhage (see Brown compaction of the lungs). There is also a ferruginization of individual muscle fibers in the uterus, nerve cells in the brain in certain mental illnesses (idiocy, early and senile dementia, Pick's atrophy, some hyperkinesias). These formations are impregnated with colloidal iron, and ferruginization can be detected only with the help of special reactions.
To detect ionized iron in tissues, the reaction of formation of turnbull blue according to Tiermann-Schmelzer to detect Fe (II) and the reaction of formation of Prussian blue [Perls method using Fe (III)] are most widely used.
The reaction for the formation of turnbull blue is carried out as follows: the prepared sections are placed for 1-24 hours in 10% ammonium sulfide solution to convert all of the fluid into bivalent sulfuric fluid. Then the sections thoroughly rinsed in distilled water are transferred for 10-20 minutes. in a freshly prepared mixture of equal parts of 20% solution of potassium ferricyanide and 1% solution of hydrochloric acid. Zh. is painted in a bright blue color; kernels can be finished with carmine. Use only glass needles to transfer sections.
According to the method of Perls, the sections are placed for several minutes in a freshly prepared mixture of 1 hour 2% aqueous solution of potassium ferricyanide and 1.5 hours 1% solution of hydrochloric acid; then the sections are rinsed with water and the kernels are stained with carmine. J. is painted blue. SH-ferritin is detected using cadmium sulfate (N. D. Klochkov).
Bibliography: Biochemical research methods in the clinic, ed. A. A. Pokrovsky, p. 440, M., 1969; In e r b about l the island and the p. A. and At t e sh e in A. B. Iron in an animal organism, Alma-Ata, 1967, bibliogr.; Glinka N. L. General chemistry, p. 682, L., 1973; Kassirsky I. A. and Alekseev G. A. Clinical hematology, p. 168, M., 1970, bibliogr.; Levin V.I. Production of radioactive isotopes, p. 149, M., 1972; Mashkovsky M. D. Medicines, part 2, p. 94, Moscow, 1977; Normal hematopoiesis and its regulation, ed. N. A. Fedorova, p. 244, M., 1976; Petrov V. N. and Shcherba M. M. Identification, prevalence and geography of iron deficiency, Klin, medical, t. 20, 1972, bibliogr.; P Ya-bov S. I. and Shostka G. D. Molecular genetic aspects of erythropoiesis, L., 1973, bibliogr.; Shch erb and M. M. Iron deficiency states, L., 197 5; Klinische Hamatologie, hrsg. v. H. Begemann, S. 295, Stuttgart, 1970; Pharmacological basis of therapeutics, ed. by L. S. Goodman a. A. Gilman, L., 1975.
G. E. Vladimirov; G. A. Alekseev (gem.), V. V. Bochkarev (rad.), A. M. Vikhert (stalemate. an.), V. V. Churyukanov (farm.).
How the material became known from 3-4 thousand BC. e. At first, meteoritic iron fell into the field of view of man, so that in those days it was valued more than gold. Then the Hittites mastered the development of sedimentary deposits, and the Romans learned how to smelt iron.
Since then, the use of metal has only expanded. And so today we will talk about the use of iron and its compounds in human life: in everyday life, the national economy, industry and the use of metal in other areas.
So, let's find out why iron has received the greatest use in metallurgy.
Under iron, they often do not mean a substance as such, but low-carbon electrical steel - this is the name of a metal alloy according to GOST. Really pure iron is not easy to obtain, and it is used exclusively for the production of magnetic materials.
Iron is ferromagnetic, that is, it becomes magnetized in the presence of a magnetic field. However, this property is highly dependent on impurities and the structure of the metal. absolute pure iron is 100-200 times higher than the same indicators of technical steel. The same can be said about the grain size: the larger the grain, the better the magnetic properties of the substance. Machining also matters, although its influence is not so impressive. Only such iron is used to obtain all magnetic materials for electrical engineering and magnetic drives.
In all other areas of the national economy, steel and cast iron find their use, so that, speaking of the use of iron, one speaks of the use of steel.
The video below will tell about the methods of using iron alloys:
Connections
All metals used in production are divided into non-ferrous and ferrous. Black - these are iron alloys, in particular, steel and cast iron, the rest - silver, are non-ferrous. Accordingly, those involved in the smelting of iron and steel are called ferrous metallurgy, and all the rest are called non-ferrous. Ferrous metallurgy accounts for 95% of all metallurgical processes. Ferrous alloys are divided in this way:
- steel- an alloy of iron with carbon and other ingredients, whose mass fraction does not exceed 2.14%. Carbon gives steel its ductility and hardness. The composition may also include manganese, phosphorus, sulfur, and so on;
- cast iron- an alloy with carbon, where a higher content of the element is allowed - up to 4.3%. Moreover, cast irons differ in their properties depending on the form in which the alloy contains carbon: if the substance has reacted with iron, white cast iron is obtained, if included in the form of graphite - gray;
- ferrite- iron with a minimum admixture of carbon and other elements - 0.04%. Actually, this is chemically pure iron;
- perlite- not an alloy, but a mechanical mixture of iron carbide and ferrite. Its properties differ markedly from those of a metal;
- austenite- a solution of carbon in iron with a share of the first up to 0.8%. Austenite is ductile and has no magnetic properties.
Read about the methods of using iron in the form of steel below.
Become
Of course, steel and cast iron are most widely used, and their use depends on the proportion of carbon in the composition. On this basis, carbon and alloy steels are distinguished. In the first case, impurities are permanent, that is, they enter the alloy due to the peculiarities of the smelting process. Doped additives are introduced specifically to impart special properties to the material. As alloying elements, vanadium, chromium, and so on are used.
Carbon steels are divided into 3 groups:
- low carbon- the share of the element is less than 0.25%, the most malleable and ductile;
- medium carbon- with a share of carbon up to 0.6%;
- high carbon– element content exceeds 0.6%.
Alloy steels also make up 3 groups:
- low-alloyed– mass fraction of all components is 2.5%:
- medium alloyed– here the total content can reach 10%;
- highly alloyed– the proportion of alloying elements exceeds 10%.
Alloy steels are usually the material for tools and machine components, since the addition of additional ingredients increases the strength of the alloy, gives it heat resistance or corrosion resistance. Carbonaceous materials are mainly used for frame structures, the manufacture of plumbing, and so on.
All steels can be divided by purpose:
- construction- mainly high or medium carbon steels. Alloys are used for all construction works: from the construction of metal frames to the manufacture of household items and roofing sheets;
- structural- low-carbon steels with an element fraction of up to 0.75%. It is a material for all branches of mechanical engineering - from bicycles to marine vessels;
- instrumental- low-carbon, but differs from the structural one also by a very low manganese content - no more than 0.4%. This is the basis of the measuring, stamped, cutting tool;
- special steels- are divided into 2 subspecies: with special physical qualities - electrical steel with specified magnetic properties, and with special chemical - heat-resistant, stainless, and so on.
The use of alloy steels is determined by their qualities.
- So, stainless steel is used in construction and mechanical engineering, where higher than usual resistance to corrosion is required.
- Heat-resistant alloys "work" at high temperatures - turbines, heating lines. Heat-resistant - do not oxidize at high temperatures, which is important for many working units in heat engineering.
Another division of alloys is by quality. This parameter determines the content of phosphorus and sulfur - harmful impurities that reduce the strength of the alloy. There are 4 types:
- ordinary quality steel includes up to 0.06% sulfur and 0.07% phosphorus. These are common building materials used in the manufacture of pipes, channels, angles, profiles and other metal products;
- quality- allows a sulfur content of up to 0.035% and the same proportion of phosphorus. It is also used in the production of rolled metal products, housings, machine parts and some grades of tool steel;
- high quality– the share of sulfur and phosphorus does not exceed 0.025%, respectively. This category includes tool and structural steels used under high load conditions;
- especially high quality– sulfur content is less than 0.015%, phosphorus content is less than 0.025%. This material is characterized by maximum wear resistance. Some grades stand out in a special category and are marked accordingly, for example, ball-bearing steel, or high-speed cutting steel - an indispensable element of a high-quality cutting tool.
The video below will tell about the use of cast iron and steel:
Cast iron
The use of cast iron is not much less, since its mechanical qualities are quite comparable to many steel grades. In accordance with the category of cast iron, the application also differs:
- gray cast iron- carbon in iron is in the form of graphite plates. It has good casting properties and low shrinkage. But its most remarkable quality is its resistance to variable loads. Gray cast iron is used in the manufacture of rolling machines, beds, bearings, flywheels, piston rings, parts of tractor and automobile engines, housings, and so on;
- white cast iron- carbon is bonded to iron. Almost entirely used for steel;
- ductile iron- carbon is in the form of spherical inclusions. This form provides high resistance to tensile and bending loads. Cast iron is used to make turbine parts, tractor and car crankshafts, gears, molds, and so on.
Cast iron can also be alloyed to produce an alloy with a variety of properties.
- Wear-resistant cast iron is used for the manufacture of pump parts, brakes, clutch discs.
- Heat-resistant is used in the construction of blast furnaces, open-hearth, thermal furnaces.
- Heat-resistant is used in the construction of gas furnaces, in the manufacture of compressor equipment, diesel engines.
Use in construction
 Steel and cast iron combine strength, durability and affordability in a unique way. Therefore, it is not possible to replace it with any other structural material. In construction, rolled metal products are basic along with concrete and bricks.
Steel and cast iron combine strength, durability and affordability in a unique way. Therefore, it is not possible to replace it with any other structural material. In construction, rolled metal products are basic along with concrete and bricks.
capital construction
Metal can be given any shape: from the simplest - a rod, to the bizarrely complex - wrought iron. In construction, they find application for all options.
In addition to the fact that steel itself is durable, especially after special processing, another feature is actively used in this area. The fact is that profile metal products are in no way inferior in strength to a solid part of the same size and shape. And this significantly reduces the material consumption of building elements, reduces their cost, reduces weight, and so on. In construction, this combination is extremely important.
Used rolled metal products are divided into 3 main groups.
- Shaped - channels, I-beams, angular and regular profile, as well as perforated. This also includes a special profile used, for example, in mine workings. Shaped metal is used in the construction of all types of frames for any structure - from buildings to bridges and dams. It is also used, if necessary, to strengthen the structure.
- High-quality - fittings, beams, pipes, circles and more. These elements are used almost more often than shaped and are very diverse:
- reinforcement - steel bars of different diameters, smooth and with ribs. The reinforcement is designed to increase the strength of the building, and the indicator is not only resistance to stationary load, but also an increase in strength under tensile and bending loads. Reinforcement is used in the construction of foundations, ceilings, strengthening walls, as well as in strengthening other structural units - stairs, for example;
- pipes - both round and profile are used. Rectangular square pipes are preferable, since their welding and fastening is easier than in the case of round ones, and the load resistance is the same;
- a beam is a variant of a solid cast product when strength is required under the highest loads.
- Rolled sheets - hot and cold rolled sheets with and without coating. These are roofing sheets, and so on. Decking is used not only for roofing, but also for the construction of various fences, since the material combines relative lightness with high strength and resistance to temperature extremes.
Stainless steels for sheet metal are rarely used, since the cost of the alloy is higher.
Finishing work
They are often based on metal products - pipes, profiles, and sheet metal.
- Pipes of unusual shapes are actively used in modern interiors. They are used to construct sleeping blocks, ceilings and partitions in the room, fences, both stair and street, and are even used in the manufacture of furniture. Here, pipes, of course, are selected with a beautiful coating - chrome, although there are also painted products.
- Profile - niches and decorative ledges, columns and ceilings, wall and fireplace decoration, and so on and so forth. Everything that is sheathed and lined with drywall, film, clapboard, panels - absolutely everything has a frame made of a metal profile. In the manufacture of furniture - wardrobes, for example, a specialized profile is also used. Steel is much stronger and more durable than steel.
- Metal can act not only as a frame, but as a finishing material. Slatted, cassette, panel ceilings are exceptionally diverse, interesting and durable. Both slats and panels can be made from but if a durable and strong solution is required - for example, for finishing the ceiling of a railway station, where vibration resistance is required, steel is of course used.
- Doors - they no longer belong to the finishing work, but rather act as an element of the protection system. Entrance doors made of steel of sufficient thickness are the most popular and reliable way to prevent home break-ins. The same can be said about garage doors, for example, or gates to the yard.
- Stair structures - metal stairs are very diverse: from attached or folding attic, to a capital structure on the 2nd floor. This option is durable and reliable, while it can be very beautiful. Modern modular stairs are combined with glass, transparent plastic or even wood, and wrought iron railings can decorate a stone staircase.
Communications
 Despite the fact that the steel pipeline is actively replacing plastic and metal-plastic ones, it is still extremely far from completely surrendering positions. The reason is simple: there is little that compares to the strength and durability of steel.
Despite the fact that the steel pipeline is actively replacing plastic and metal-plastic ones, it is still extremely far from completely surrendering positions. The reason is simple: there is little that compares to the strength and durability of steel.
- Water supply and sewerage - if plastic products can be connected to service a private house or apartment, then this cannot be said about the main line and even the pipeline serving an apartment building. Only iron pipes are allowed, and they comply with firmly established standards.
- Gas pipeline - no options, only steel is used.
- Heating systems - in a building, the system may include plastic pipes. City and regional highways, not to mention the pipeline directly serving the boiler room, can only be iron. The initial temperature of the heated water is much higher than that which plastic conduits can withstand, not to mention the pressure.
- Batteries and radiators, as a rule, are also used iron or cast iron - cast iron has a higher heat capacity and resistance to water hammer. No matter what modern options heaters are replaced with, steel is still present in the structure. Electric radiators - convector, oil, are always made of steel, since the latter, having high thermal conductivity, instantly gives off heat to the air.
- Cables - wiring in the house is most often hidden in plastic boxes. However, power cables with a large cross section are protected by metal pipes.
- Chimneys - steel pipes are the simplest, most affordable and lightest option. For their manufacture, special heat-resistant steel is used, and it is resistant to corrosion.
Equipment and household items
Any appliances installed in the house are made of steel.
- Heating boilers - no matter what fuel the devices work on, their bodies are always made of steel. Solid fuel stoves have cast iron parts.
- Kitchen equipment - stoves, ovens, microwaves, steamers and so on have steel bodies and parts. In the kitchen, steel is also a sought-after finishing material: worktops, for example, finishing an apron. Steel is a very decorative material and only seems simple.
- Washing machines, dryers and dishwashers are also not complete without iron.
- Steel plumbing is rarely used due to its high thermal conductivity, but cast-iron bathtubs and washbasins are still being installed. The material retains heat better and is very durable.
- Crockery and cutlery, coasters and vases, holders and fittings, electrical equipment and small accessories - the places where iron is not used can be counted on the fingers.
- Wrought iron - decorative objects of this kind are a real work of art, especially when it comes to hot forging, in which each product, each detail is made by hand and only once. Forged gratings, railings, fireplaces, fences adorn palaces and modern pavilions, and, of course, residential apartments.
Iron is the main structural material. In construction, steel and cast iron are basic materials along with building stone. The application and variety of alloys defies description.
Even more useful information on the use of iron is contained in this video:
The benefits of iron for the body
The main function of iron in the body is considered to be the formation of hemoglobin. This is not surprising, because it contains three-fourths of iron reserves. But in the composition of other protein structures, the percentage of iron is relatively low - about 5%.
Why is hemoglobin needed? A protein containing a large amount of iron binds oxygen molecules that are transported with blood to working tissues and organs. That is why a decrease in the amount of hemoglobin in the blood immediately affects the overall well-being and performance. So even a slight loss of blood is fraught with disorders for the body. For athletes, iron deficiency is fraught with impaired recovery after intense physical activity.
Other functions of iron include:
- Energy supply of muscles. The “cheapest” source of fuel for muscles is oxygen. Thanks to its transformation in the process of a series of chemical reactions, the muscle receives energy for contraction. In addition to oxygen, other sources of energy are also used. These are phosphates contained in cells - creatine phosphate and ATP, as well as muscle and liver glycogen. However, their reserves are too small to support work lasting more than 1 minute. Creatine phosphate is enough for work lasting up to 10 seconds, ATP - for 2-3 seconds. The higher the concentration of hemoglobin in the blood, the more oxygen it is able to supply to the working tissues and organs. But iron deficiency can cause muscle spasms, which increase during periods of rest (sleep, sitting).
- Energizing the brain. Oxygen is necessary for the brain as well as for the muscles. Moreover, iron deficiency is fraught with the development of Alzheimer's disease, dementia (acquired dementia) and other diseases caused by impaired brain activity.
- Body temperature regulation. This function is performed indirectly by iron. The stability of the concentration of iron in the blood determines the adequacy of the flow of all metabolic processes.
- Strengthening immunity. The microelement is necessary for hematopoiesis. White (lymphocytes) and red (erythrocytes) blood cells form in the presence of iron. The former are responsible for immunity, while the latter supply oxygen to the blood. If the amount of iron in the body corresponds to the norm, it is able to independently resist diseases. As soon as the concentration of iron decreases, infectious diseases make themselves felt.
- Fetal development. During pregnancy, it is important to consume a sufficient amount of iron, since part of it is consumed during hematopoiesis in the fetus. But iron deficiency increases the risk of preterm birth, provokes underweight in the newborn and developmental disorders.
How iron interacts in the body
By itself, the normal concentration of iron in the body does not guarantee good health, high immunity, the absence of diseases and performance. No less important is the interaction of this trace element with other substances, because the functions of some can adversely affect the functions of others.
Avoid combining iron with:
- vitamin E and phosphates: iron absorption is disturbed;
- Tetracycline and fluoroquinolones: the process of absorption of the latter is inhibited;
- Calcium: the process of iron absorption is disturbed;
- milk, coffee and tea - iron absorption worsens;
- zinc and copper - the process of absorption in the intestine is disrupted;
- soy protein - absorption is suppressed;
- chromium: iron inhibits its absorption.
But ascorbic acid, sorbitol, fructose and succinic acid improve the absorption of iron by the body.
These nuances must be taken into account while taking iron-containing preparations, since instead of improving well-being, you can get the opposite effect.
The role of iron in the occurrence and course of various diseases
There are many diseases in which the use of foods rich in iron can exacerbate the situation.
People with elevated levels of iron in the body are at greater risk of infections, heart disease, and certain types of cancer (especially men).
In the form of free radicals, iron provokes the development of atherosclerosis. The same goes for rheumatoid arthritis. The use of iron in this disease provokes inflammation of the joints.
With individual intolerance to iron, the use of certain foods causes heartburn, nausea, constipation and diarrhea.
During pregnancy, an excess of iron increases the risk of developing placental pathology (free radical oxidation increases, as a result of which mitochondria, the oxygen “depots” of cells, die).
With pathological disorders of iron absorption, the risk of hemochromatosis disease is increased - the accumulation of iron in the internal organs (liver, heart, pancreas).
What foods contain iron

Iron stores are replenished at the expense of animal and vegetable products. The first contain "heme" iron, the second - "non-heme".
For the assimilation of heme, animal products are used - veal, beef, pork, rabbit meat and offal (liver, kidneys). To benefit from non-heme foods, you need to consume vitamin C along with iron-containing foods.
The following products of plant origin, mg Fe2+, are considered record holders for iron content:
- peanuts - 200 g of the product contains 120;
- soybeans - in 200 g of the product - 8.89;
- potatoes - in 200 g of the product - 8.3;
- white beans - in 200 g of the product - 6.93;
- beans - in 200 g of the product - 6.61;
- lentils - in 200 g of the product - 6.59;
- spinach - in 200 g of the product - 6.43;
- beets (tops) - in 200 g of the product - 5.4;
- chickpeas - in 100 g of the product - 4.74;
- Brussels sprouts - in 200 g of the product - 3.2;
- white cabbage - in 200 g of the product - 2.2;
- green peas - in 200 g of the product - 2.12.
Of the cereals in the diet, it is better to include oatmeal and buckwheat, wholemeal flour, wheat germ. From herbs thyme, sesame (sesame). A lot of iron is found in dried porcini mushrooms and chanterelles, apricots, peaches, apples, plums, quince. As well as figs, pomegranate and dried fruits.
Among the products of animal origin, iron stores in beef kidneys and liver, fish, eggs (yolk). In meat products - veal, pork, rabbit, turkey. Seafood (clams, snails, oysters). Fish (mackerel, pink salmon).
Iron absorption
Interestingly, when eating meat products, iron is absorbed by 40-50%, while eating fish products - by 10%. The record holder for the absorption of iron is the liver of animals.
From plant products, the percentage of iron that is absorbed is even less. From legumes, a person absorbs up to 7%, from nuts - 6, from fruits and eggs - 3, from cooked cereals - 1.
Advice! Benefits for the body is a diet that combines products of plant and animal origin. When adding 50 g of meat to vegetables, the absorption of iron doubles. With the addition of 100 g of fish - three times, with the addition of fruits containing vitamin C - five times
How to keep iron in food and its combination with other substances

When cooking, foods lose some of their nutrients, and iron is no exception. Iron in animal products is more resistant to heat. With vegetables and fruits, everything is more complicated - part of the iron passes into the water in which the food is cooked. The only way out is to minimize the heat treatment of plant products.
To increase the absorption of iron, consume iron-rich foods along with vitamin C. Half a grapefruit or orange is enough for the body to absorb three times more iron. The only caveat is that this rule only applies to iron-containing products of plant origin.
Vitamin A is required in the diet, the lack of which blocks the body's ability to use iron stores to form erythrocytes (red blood cells).
With a lack of copper, iron loses its "mobility", as a result of which the process of transporting useful substances from "storage" to cells and organs is disrupted. To avoid this, include more legumes in your diet.
The combination of iron with B vitamins: the "working capacity" of the latter is greatly enhanced.
But dairy foods and grains are best consumed separately from iron-containing foods, as they block the absorption of the trace element in the intestine.
Daily intake of iron
- up to 6 months - 0.3;
- 7-11 months - 11;
- up to 3 years - 7;
- up to 13 years - 8-10.
Teens:
- from 14 to 18 years old (boys) - 11; girls - 15.
Adults:
- men - 8-10;
- women under 50 - 15-18; over 50 years old - 8-10, pregnant women - 25-27.
What is the danger of iron deficiency in the body
Iron deficiency in the body is dangerous in the following condition:
- acute anemia, or anemia - a decrease in the concentration of hemoglobin in the blood, in which the number of red blood cells also decreases and their qualitative composition changes. The result of anemia is a decrease in the respiratory function of the blood and the development of oxygen starvation of tissues. Acute anemia can be recognized by the pallor of the skin and increased fatigue. Weakness, regular headache and dizziness are signs of iron deficiency. Tachycardia (rapid heartbeat) and shortness of breath are harbingers of problems with the heart and lungs;
- fatigue and weakness in the muscles;
- excessive menstrual bleeding in women.
Iron deficiency in the body leads to deterioration of the skin, brittle nails, hair loss. Memory impairment, increased irritability are signs of iron deficiency. Decreased efficiency and constant drowsiness are harbingers of oxygen starvation.
Iron deficiency can be caused by the following factors:
- increased blood loss. The root cause of this scenario may be a donor blood transfusion, profuse bleeding in women and soft tissue damage;
- intense physical activity of aerobic and aerobic-strength orientation (those that develop endurance). During such exercises, red blood cells have to carry oxygen faster, as a result of which the daily consumption of hemoglobin can almost double;
- active mental activity. During creative work, not only iron reserves are actively consumed, but also glycogen stored in the liver and muscles;
- diseases of the gastrointestinal tract: gastritis with low acidity, duodenal ulcer, cirrhosis of the liver, autoimmune bowel diseases provoke poor absorption of iron.
How to quickly fill the lack of iron
To compensate for iron deficiency in the body, nutritionists recommend eating foods of plant and animal origin. The former are a source of so-called "non-heme" iron, that is, iron that is not part of hemoglobin. In such products, iron is usually combined with vitamin C.
Non-heme foods such as legumes and green leafy vegetables, as well as whole grains, are best for iron deficiency.
Heme products contain iron, which is part of hemoglobin. The largest reserves of hemoglobin are characteristic of all food of animal origin, as well as seafood. Unlike “non-heme” foods, “heme” foods replenish iron stores faster, as the body absorbs them more easily.
Advice! Despite the fact that "heme" products are absorbed by the body faster, you should not get carried away with them too much. For iron replenishment, it is best to combine plant and animal foods, such as green leafy vegetables and red meats.
However, it is important to remember the secrets of cooking, because the final percentage of iron in food depends on the cooking methods. For example, whole grains lose about 75% of their iron reserves during processing. This is why whole grain flour has little to no benefit to the body. Approximately the same thing happens when cooking food of plant origin by cooking - part of the iron remains in the water. If you cook spinach for 3 minutes, no more than 10% of iron reserves will remain.
If you want to get the most benefit from plant-based foods, try to avoid long-term cooking and minimize the amount of water. The ideal cooking method is steaming.
With animal products, everything is much simpler - iron, which is part of hemoglobin, is highly resistant to heat treatment.
What you need to know about excess iron in the body

It would be unfair to believe that the danger to health is only a lack of iron. Its excess is also fraught with unpleasant symptoms. Due to the excessive accumulation of iron in the body, the work of many functional systems is disrupted.
Reasons for an overdose. Most often, the cause of an increased concentration of a microelement is a genetic failure, as a result of which the absorption of iron by the intestine increases. Less often - blood transfusion in large quantities and uncontrolled use of iron-containing drugs. The latter happens with an independent increase in the dose of an iron-containing drug when the next dose is missed.
With an excess of iron in the body, this usually happens:
- skin pigmentation changes (symptoms are often confused with hepatitis) - palms, armpits turn yellow, old scars darken. The sclera, palate of the oral cavity and tongue also acquire a yellowish tint;
- the heart rhythm is disturbed, the liver enlarges;
- appetite decreases, fatigue increases, headache attacks become more frequent;
- the activity of the digestive organs is disrupted - nausea and vomiting alternate with diarrhea, aching pain appears in the stomach area;
- immunity decreases;
- the likelihood of developing infectious and neoplastic pathologies, for example, cancer of the liver and intestines, as well as the development of rheumatoid arthritis, increases.
Preparations containing iron
Iron preparations include medicines containing salts and complexes of microelement compounds, as well as its combinations with other minerals.
In order to avoid pathological conditions and complications, iron-containing preparations should be taken only as prescribed by a doctor after a series of tests. Otherwise, excess iron can lead to disruption of the heart, liver, stomach, intestines and brain.
- washed down with a small amount of water;
- incompatible with calcium preparations, Tetracycline, Levomycetin, as well as antacids (Almagel, Phosphalugel, etc.);
- taken in strict dosage. If for some reason the next dose of the drug was missed, the next dose remains unchanged. An overdose of iron (300 milligrams per day) can be fatal;
- the minimum course is two months. During the first month, hemoglobin and red blood cells return to normal. In the future, taking drugs is aimed at replenishing iron stores (filling the "depot"). The dosage during the second month is reduced.
It should be remembered that even if all precautions are taken, taking iron-containing preparations can cause such side effects as skin flushing, nausea, loss of appetite, drowsiness, headache, disruption of the digestive system (constipation, diarrhea, intestinal colic, heartburn and belching), metallic taste in the mouth. In some cases, teeth may darken (the oral cavity contains hydrogen sulfide, which, when interacting with iron, is converted into iron sulfide).
Advice! To avoid darkening of the teeth (especially true for caries), immediately after taking iron-containing preparations, the oral cavity must be rinsed. If the drug is available in liquid dosage form, it is best to take it through a tube. If any of these symptoms appear, the medication should be stopped immediately.
An overview of iron supplements is provided below.
Among the most commonly prescribed iron preparations are Conferon, Ferakryl, Ferrum lek, Hemostimulin. Their advantages are the most accurate dosage and a minimum of side effects.
The dosage of the drug is calculated individually - 2 mg per 1 kg of the patient's body weight (but not more than 250 mg per day). For better absorption, drugs are taken with food, with a small amount of liquid.
Positive changes (an increase in the number of reticulocytes) are diagnosed within a week after the start of taking the funds. After another two to three weeks, the concentration of hemoglobin increases.
| A drug | Release form | Compound |
| Hemopherprolongatum | Film-coated tablets, weighing 325 mg | Ferrous sulfate, in one tablet - 105 mg Fe2 + |
| Tardyferon | Prolonged release tablets | Mucoproteosis and ascorbic acid, in one tablet - 80 mg Fe2 + |
| Ferrogluconate and Ferronal | 300 mg tablets | Ferrous gluconate, in one tablet - 35 mg Fe2 + |
| Ferrogradumet | Coated tablets | Ferrous sulfate plus a plastic matrix - gradumet, in one tablet - 105 mg Fe2 + |
| Heferol | Capsules 350 mg | Fumaric acid, in one tablet - 100 mg Fe2 + |
| Aktiferrin | Capsules, oral drops, syrup | Ferrous sulfate, D, L-serine (capsules and oral drops) and ferrous sulfate, D, L-serine, glucose, fructose, potassium sorbate (syrup). In 1 capsule and 1 ml of syrup - 38.2 mg of Fe2 +, in 1 ml of drops, in 1 ml of syrup - and 34.2 mg of Fe2 + |
| Gemsineral-TD | Capsules | Microgranules of ferrous fumarate, folic acid, cyanocobalamin. One capsule contains 67 mg Fe2+ |
| Gino-Tardiferon | Tablets | Ferrous sulfate, folic and ascorbic acids, mucoproteosis. One tablet contains 80 mg Fe2+ |
| Globiron | Gelatin capsules 300 mg | Ferrous fumarate, vitamins B6, B12, folic acid, sodium docusate. One capsule contains 100 mg Fe2+ |
| Ranferon-12 | Capsules 300 mg | Ferrous fumarate, ascorbic and folic acids, cyanocobalamin, zinc sulfate, iron ammonium citrate. One capsule contains 100 mg Fe2+ |
| Sorbiferdurules | Film-coated tablets with prolonged release of iron ions | Ferrous sulfate, ascorbic acid, matrix (durules). One tablet contains 100 mg Fe2+ |
| Totem | Solution for oral administration in 10 ml ampoules | Iron gluconate, manganese, copper, as well as benzoate, sodium citrate and sucrose. One ampoule contains 50 mg Fe2+ |
| Heferol | Capsules 350 mg | Fumaric acid. One capsule contains 100 mg Fe2+ |
| Fenyuls | Capsules | Iron sulfate, folic and ascorbic acids, thiamine. As well as riboflavin, cyanocobalamin, pyridoxine, fructose, cysteine, calcium pantothenate, yeast. One capsule contains 45 mg Fe2+ |
Contraindications to taking iron supplements
|
|
|
|
|
|
Conditional contraindications: |
|
|
|
Iron injections and their features are described below. In addition to iron-containing capsules and tablets, injections are prescribed. Their reception is necessary for:
- chronic pathologies of the digestive organs, accompanied by reduced absorption of iron. Diagnoses: pancreatitis (inflammation of the pancreas), malabsorption syndrome, celiac disease, enteritis;
- ulcerative colitis of a nonspecific nature;
- intolerance to iron salts or hypersensitivity with allergic manifestations;
- peptic ulcer of the stomach and duodenum during periods of exacerbation;
- postoperative period after removal of part of the stomach or small intestine.
The advantage of injections is the rapid and maximum iron saturation compared to other forms of drug release.
Important! When taking tablets and capsules, the maximum dose should not exceed 20-50 mg (when taking 300 mg of iron, a fatal outcome is possible). When injected, the maximum dose is considered to be 100 mg of an iron preparation.
Side effects when iron is administered by injection: seals (infiltrates) of tissues at the injection site, phlebitis, abscesses, an allergic reaction (in the worst case, anaphylactic shock immediately develops), DIC, iron overdose.
Varieties of drugs are given in the table
| A drug | Release form | Compound |
| Ferrum Lek (intramuscularly) | Ampoules of 2 ml | Iron hydroxide and dextran. One ampoule contains 100 mg Fe2+ |
| Venofer (intravenously) | Ampoules of 5 ml | Iron hydroxide sucrose complexes. One ampoule contains 100 mg Fe2+ |
| Ferkoven (intravenously) | Ampoules 1 ml | Iron saccharate, carbohydrate solution and cobalt gluconate. One ampoule contains 100 mg Fe2+ |
| Zhektofer (intramuscularly) | Ampoules of 2 ml | Iron-sorbitol-citric-acid complex |
| Ferrlecit (solution - intramuscularly, ampoules - intravenously) | Solution for injection in ampoules of 1 and 5 ml | Iron gluconate complex |
| Ferbitol (intramuscularly) | Ampoules 1 ml | Iron sorbitol complex |