What does physics pressure depend on? Pressure. How is pressure measured? Why does the Earth's air envelope exist?
Why doesn't a person standing on skis fall into the loose snow? Why does a car with wide tires have better cross-country capability than a car with regular tires? Why does a tractor need tracks? We will learn the answer to these questions by becoming familiar with the physical quantity called pressure.
Solid pressure

When a force is applied not to one point of the body, but to many points, then it acts on the surface of the body. In this case, we talk about the pressure that this force creates on the surface of a solid body.
In physics, pressure is a physical quantity that is numerically equal to the ratio of the force acting on a surface perpendicular to it to the area of this surface.
p = F/S ,
Where R - pressure; F - force acting on the surface; S - surface area.
So, pressure occurs when a force acts on a surface perpendicular to it. The amount of pressure depends on the magnitude of this force and is directly proportional to it. The greater the force, the greater the pressure it creates per unit area. An elephant is heavier than a tiger, so it exerts more pressure on the surface. A car presses on the road with more force than a pedestrian.
The pressure of a solid is inversely proportional to the surface area on which the force acts.
Everyone knows that walking in deep snow is difficult because your feet constantly sink. But it's quite easy to do it on skis. The whole point is that in both cases a person acts on the snow with the same force - gravity. But this force is distributed over surfaces with different areas. Since the surface area of the skis is larger than the area of the soles of the boots, the person’s weight in this case is distributed over a larger area. And the force acting per unit area turns out to be several times smaller. Therefore, a person standing on skis puts less force on the snow and does not fall into it.
By changing the surface area, you can increase or decrease the amount of pressure.
When going on a hike, we choose a backpack with wide straps to reduce pressure on the shoulder.
To reduce the pressure of the building on the ground, the area of the foundation is increased.
Truck tires are made wider than passenger car tires so that they exert less pressure on the ground. For the same reason, a tractor or tank is made on caterpillar tracks, and not on wheels.
Knives, blades, scissors, and needles are sharpened so that they have the smallest possible cutting or piercing area. And then even with the help of a small applied force, a lot of pressure is created.
For the same reason, nature provided animals with sharp teeth, fangs, and claws.
Pressure is a scalar quantity. In solids it is transmitted in the direction of the force.
The unit of force is newton. The unit of area measurement is m2. Therefore, the unit of measurement for pressure is N/m2. This quantity in the international system of SI units is called pascal (Pa or Ra). It got its name in honor of the French physicist Blaise Pascal. A pressure of 1 pascal is caused by a force of 1 newton acting on a surface measuring 1 m2.
1 Pa = 1 N/m2 .
Other systems use units such as bar, atmosphere, mmHg. Art. (millimeters of mercury), etc.
Pressure in liquids
If in a solid body pressure is transmitted in the direction of the force, then in liquids and gases, according to Pascal’s law, “ any pressure exerted on a liquid or gas is transmitted in all directions without change ».
Let's fill a ball with tiny holes connected to a narrow tube in the form of a cylinder with liquid. Let's fill the ball with liquid, insert a piston into the tube and start moving it. The piston presses on the surface of the liquid. This pressure is transmitted to every point in the fluid. Liquid begins to pour out of the holes in the ball.
Filling the ball with smoke, we will see the same result. This means that in gases pressure is also transmitted in all directions.
A liquid, like any body on the surface of the Earth, is affected by gravity. Each layer of liquid in the container creates pressure with its weight.
This is confirmed by the following experience.

If you pour water into a glass vessel with a rubber film instead of a bottom, the film will bend under the weight of the water. And the more water there is, the more the film will bend. If we gradually immerse this vessel with water in another container, also filled with water, then as it descends the film will straighten. And when the water levels in the vessel and the container are equal, the film will straighten out completely.

At one level, the pressure in the liquid is the same. But with increasing depth it increases, since the molecules of the upper layers exert pressure on the molecules of the lower layers. And they, in turn, put pressure on the molecules of the layers located even lower. Therefore, at the lowest point of the container the pressure will be highest.
Pressure at depth is determined by the formula:
p = ρ g h ,
Where p - pressure (Pa);
ρ - liquid density (kg/m3);
g - free fall acceleration (9.81 m/s);
h - height of the liquid column (m).
From the formula it is clear that pressure increases with increasing depth. The lower a submersible goes in the ocean, the more pressure it will experience.
Atmosphere pressure

Evangelista Torricelli
Who knows, if in 1638 the Duke of Tuscany had not decided to decorate the gardens of Florence with beautiful fountains, atmospheric pressure would not have been discovered in the 17th century, but much later. We can say that this discovery was made by accident.
In those days, it was believed that water would rise behind the pump piston because, as Aristotle stated, “nature abhors a vacuum.” However, the event was not a success. The water in the fountains actually rose, filling the resulting “void,” but it stopped at a height of 10.3 m.
They turned to Galileo Galilei for help. Since he could not find a logical explanation, he instructed his students - Evangelista Torricelli And Vincenzo Viviani conduct experiments.
Trying to find the reason for the failure, Galileo's students found out that different liquids rise behind the pump to different heights. The denser the liquid, the lower the height it can rise. Since the density of mercury is 13 times greater than the density of water, it can rise to a height 13 times less. That's why they used mercury in their experiment.

In 1644 the experiment was carried out. A glass tube was filled with mercury. Then it was tipped into a container also filled with mercury. After some time, the column of mercury in the tube rose. But he didn't fill the whole tube. There was empty space above the column of mercury. It was later called the “Torricellian void.” But mercury did not pour out of the tube into the container either. Torricelli explained this by the fact that atmospheric air presses on the mercury and holds it in the tube. And the height of the mercury column in the tube shows the magnitude of this pressure. This was the first time atmospheric pressure was measured.
The Earth's atmosphere is its shell of air held near it by gravitational attraction. The molecules of the gases that make up this shell move continuously and chaotically. Under the influence of gravity, the upper layers of the atmosphere press on the lower layers, compressing them. The lowest layer, located at the surface of the Earth, is the most compressed. Therefore, the pressure there is the greatest. According to Pascal's law, it transmits this pressure in all directions. It is experienced by everything that is on the surface of the Earth. This pressure is called atmospheric pressure .
Since atmospheric pressure is created by the overlying layers of air, it decreases with increasing altitude. It is known that high in the mountains it is less than at the foot of the mountains. And deep underground it is much higher than on the surface.
Normal atmospheric pressure is considered to be a pressure equal to the pressure of a column of mercury 760 mm high at a temperature of 0 o C.
Atmospheric pressure measurement

Since atmospheric air has different densities at different altitudes, the value of atmospheric pressure cannot be determined using the formulap = ρ · g · h . Therefore, it is determined using special devices called barometers .
There are liquid barometers and aneroids (liquid-free). The operation of liquid barometers is based on changes in the liquid level column under atmospheric pressure.
An aneroid is a sealed container made of corrugated metal, inside of which a vacuum is created. The container contracts when atmospheric pressure increases and expands when it decreases. All these changes are transmitted to the pointer using a springy metal plate. The end of the arrow moves along the scale.
By changing the barometer readings, you can predict how the weather will change in the coming days. If the atmospheric pressure rises, then clear weather can be expected. And if it goes down, it will be cloudy.
>>Pressure and pressure force
Submitted by readers from Internet sites
A collection of physics lesson notes, abstracts on a topic from the school curriculum. Calendar thematic planning, physics 7th grade online, books and textbooks on physics. The student prepares for the lesson.
Imagine a sealed cylinder filled with air, with a piston installed on top. If you start to press on the piston, the volume of air in the cylinder will begin to decrease, air molecules will begin to collide with each other and with the piston more and more intensely, and the pressure of compressed air on the piston will increase.
If the piston is now sharply released, the compressed air will sharply push it upward. This will happen because, with a constant area of the piston, the force acting on the piston from the compressed air will increase. The area of the piston remained unchanged, but the force exerted by the gas molecules increased, and the pressure increased accordingly.
Or another example. A man stands on the ground, stands with both feet. In this position, a person is comfortable and does not experience any discomfort. But what happens if this person decides to stand on one leg? He will bend one of his legs at the knee, and will now rest on the ground with only one foot. In this position, a person will feel some discomfort, because the pressure on the foot has increased, approximately 2 times. Why? Because the area through which gravity now presses a person to the ground has decreased by 2 times. Here is an example of what pressure is and how easily it can be detected in everyday life.

From the point of view of physics, pressure is a physical quantity that is numerically equal to the force acting perpendicular to a surface per unit area of a given surface. Therefore, to determine the pressure at a certain point on the surface, the normal component of the force applied to the surface is divided by the area of the small surface element on which this force acts. And in order to determine the average pressure over the entire area, the normal component of the force acting on the surface must be divided by the total area of this surface.
Pressure is measured in pascals (Pa). This unit of measurement of pressure got its name in honor of the French mathematician, physicist and writer Blaise Pascal, the author of the fundamental law of hydrostatics - Pascal's Law, which states that the pressure exerted on a liquid or gas is transmitted to any point without changes in all directions. The pressure unit "pascal" was first introduced into circulation in France in 1961, according to the decree on units, three centuries after the death of the scientist.
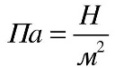
One pascal is equal to the pressure caused by a force of one newton, uniformly distributed, and directed perpendicular to a surface of one square meter.
Pascals measure not only mechanical pressure (mechanical stress), but also elastic modulus, Young's modulus, bulk modulus, yield strength, proportional limit, tensile strength, shear strength, sound pressure and osmotic pressure. Traditionally, it is in pascals that the most important mechanical characteristics of materials in strength materials are expressed.
Technical atmosphere (at), physical (atm), kilogram-force per square centimeter (kgf/cm2)
In addition to pascal, other (non-system) units are also used to measure pressure. One such unit is the “atmosphere” (at). The pressure of one atmosphere is approximately equal to the atmospheric pressure on the surface of the Earth at ocean level. Today, “atmosphere” refers to the technical atmosphere (at).

Technical atmosphere (at) is the pressure produced by one kilogram-force (kgf) distributed evenly over an area of one square centimeter. And one kilogram-force, in turn, is equal to the force of gravity acting on a body weighing one kilogram under conditions of gravitational acceleration equal to 9.80665 m/s2. One kilogram-force is thus equal to 9.80665 newton, and 1 atmosphere turns out to be equal to exactly 98066.5 Pa. 1 at = 98066.5 Pa.
For example, the pressure in car tires is measured in atmospheres; for example, the recommended tire pressure for the GAZ-2217 passenger bus is 3 atmospheres.
There is also a “physical atmosphere” (atm), defined as the pressure of a column of mercury 760 mm high at its base, given that the density of mercury is 13595.04 kg/m3, at a temperature of 0 ° C and under conditions of gravity acceleration equal to 9, 80665 m/s2. So it turns out that 1 atm = 1.033233 atm = 101,325 Pa.
As for the kilogram-force per square centimeter (kgf/cm2), this extra-systemic unit of pressure is equal to normal atmospheric pressure with good accuracy, which is sometimes convenient for assessing various effects.
The off-system unit "bar" is equal to approximately one atmosphere, but is more accurate - exactly 100,000 Pa. In the CGS system, 1 bar is equal to 1,000,000 dynes/cm2. Previously, the name “bar” was given to a unit now called “barium” and equal to 0.1 Pa or in the CGS system 1 barium = 1 dyne/cm2. The word "bar", "barium" and "barometer" all come from the same Greek word for "gravity".

The unit mbar (millibar), equal to 0.001 bar, is often used to measure atmospheric pressure in meteorology. And to measure pressure on planets where the atmosphere is very rarefied - μbar (microbar), equal to 0.000001 bar. On technical pressure gauges, most often the scale is graduated in bars.
Millimeter of mercury (mmHg), millimeter of water (mmHg)
The non-system unit of measurement “millimeter of mercury” is equal to 101325/760 = 133.3223684 Pa. It is designated “mmHg”, but is sometimes denoted “torr” - in honor of the Italian physicist, Galileo’s student, Evangelista Torricelli, the author of the concept of atmospheric pressure.
The unit was formed in connection with the convenient method of measuring atmospheric pressure with a barometer, in which the mercury column is in equilibrium under the influence of atmospheric pressure. Mercury has a high density of about 13600 kg/m3 and is characterized by a low saturated vapor pressure at room temperature, which is why mercury was chosen for barometers at one time.
At sea level, the atmospheric pressure is approximately 760 mm Hg, it is this value that is now considered normal atmospheric pressure, equal to 101325 Pa or one physical atmosphere, 1 atm. That is, 1 millimeter of mercury is equal to 101325/760 pascal.

Pressure is measured in millimeters of mercury in medicine, meteorology, and aviation navigation. In medicine, blood pressure is measured in mmHg, in vacuum technology it is graduated in mmHg, along with bars. Sometimes they even simply write 25 microns, meaning microns of mercury when we are talking about evacuation, and pressure measurements are carried out with vacuum gauges.
In some cases, millimeters of water column are used, and then 13.59 mm water column = 1 mm Hg. Sometimes this is more appropriate and convenient. A millimeter of water column, like a millimeter of mercury, is a non-systemic unit, equal in turn to the hydrostatic pressure of 1 mm of a water column, which this column exerts on a flat base at a water column temperature of 4 ° C.
Pressure is the ratio of the force that acts perpendicular to a surface to the area of that surface. Pressure is measured in pascals (1 Pa is the pressure that a force of 1 newton produces when applied to a surface of one square meter).
Pressure force is the force exerted by pressure on a specific surface. It is measured in newtons (1 N). The smaller the surface area on which this pressure is applied, the smaller the applied force can be with which the expected effect can be achieved.
The pressure force acts on the surface perpendicular to it. It cannot be identified with pressure. To determine pressure, you need to divide its force by the surface area on which it is applied. If the same force is applied to surfaces of different areas, then the pressure will be greater where the support area is smaller. If the pressure and surface area are known, then the pressure force can be found by multiplying the pressure by the area.
The force is always necessarily directed perpendicular to the surface on which it affects. According to the third, it is equal to its modulus.
Any force can play the role of pressure force. This could be a weight that deforms a support, or a force that presses a body to a certain surface, and so on.
When liquids come into contact with solids, they act on them with a certain force, which is called the pressure force. In everyday life, you can feel the impact of such force by covering the opening of the tap with your finger, from which water comes. If you pour mercury into a rubber balloon, you can see that its walls will begin to bulge outward. The force can also affect other fluids.
When solid bodies come into contact, an elastic force arises when their shape or volume changes. In liquids, such forces do not arise when changing shape. The lack of elasticity in relation to changes in shape determines the mobility of liquids. When liquids are compressed (their volume changes), elastic forces will manifest themselves. This is what is called pressure force. That is, if a liquid acts on other bodies in contact with it with a pressure force, then it is in a compressed state. The more compressed the fluid, the stronger the resulting pressure forces will be.
As a result of compression, the density of substances increases, so liquids have elasticity, which manifests itself in relation to their density. If the vessel is closed with a piston and a weight is placed on top, then when the piston is lowered, the liquid will begin to compress. A pressure force will arise in it, which will balance the weight of the piston with the load on it. If you continue to increase the load on the piston, the fluid will continue to be compressed, and the increasing pressure force will be aimed at balancing the load.
All liquids (to a greater or lesser extent) are compressible, so it is possible to measure the degree of their compression, which corresponds to a certain pressure force.
To reduce the pressure on the surface, if it is impossible to reduce the force, it is necessary to increase the support area. Conversely, to increase pressure, you need to reduce the area over which its force acts.
Gas molecules are not connected (or are too weakly connected) to each other by interaction force. Therefore, they move chaotically, almost freely, filling the entire volume of the vessel provided to them. In this regard, the properties of gas differ from those and depend on pressure to a much greater extent than liquids. What they have in common is that the pressure of both liquids and gases is independent of the shape of the container in which they may be placed.
Pressure- a physical quantity numerically equal to the force acting per unit surface area perpendicular to this surface. The symbol commonly used to indicate pressure is p- from lat. pressūra(pressure).
The pressure on the surface may have an uneven distribution; therefore, a distinction is made between the pressure on a local fragment of the surface and the average pressure on the entire surface.
The pressure on a local surface area is defined as the ratio of the normal component of the force dFn, acting on this fragment of the surface, to the area of this fragment dS:
The average pressure over the entire surface is the ratio of the normal component of the force Fn, acting on a given surface, to its area S:
The pressure of gases and liquids is measured using pressure gauges, differential pressure gauges, vacuum gauges, pressure sensors, and atmospheric pressure - barometers.
Units of pressure measurement have a long history and, taking into account different media (liquid, gas, solid), are quite diverse. Let's give the main ones.
Pascal
In the International System of Units ( SI) is measured in pascals (Russian designation: Pa; international: Pa). Pascal is equal to the pressure caused by a force equal to one newton, uniformly distributed over a surface normal to it with an area of one square meter.
1 Pa = 1 N/m 2
One pascal is a small pressure. Approximately this pressure is created by a piece of paper from a school notebook lying on the table. Therefore, multiple units of pressure are often used:
Then we get the following correspondence: 1 MPa = 1 MN/m² = 1 N/mm² = 100 N/cm².
Also, the scales of instruments for measuring pressure can be graduated in N/m 2 or N/mm 2 values.
Ratios of values to 1 Pa:
Dina
Dina(Russian designation: din, international designation: dyn) - a unit of force in the GHS system of units. One dyne is numerically equal to the force that imparts to a body weighing 1 gram an acceleration of one centimeter per second per second.
1 dyne = 1 g cm/s 2 = 10 -5 H = 1.0197 10 -6 kgf
GHS(centimeter-gram-second) is a system of measurement that was widely used before the adoption of the International System of Units (SI). Other name - absolute physical system of units.
Bar (bar, bar)
Bar (Russian designation: bar; international: bar;) - a non-systemic unit of pressure, approximately equal to one atmosphere, used for liquids and gases under pressure.
Why bar and not pascal? For technical measurements where high pressure is present, the pascal is too small a unit. Therefore, a larger unit was introduced - 1 bar. This is approximately the pressure of the earth's atmosphere.
Bar is a non-system unit of pressure measurement.
Kilogram-force
Kilogram-force is equal to the force that imparts to a mass at rest, equal to the mass of the international prototype of the kilogram, an acceleration equal to the normal acceleration of gravity (9.80665 m/s 2).
1 kgf = 1 kg * 9.80665 m/s 2 = 9.80665 N
A kilogram-force is approximately equal to the force with which a body weighing 1 kilogram presses on a scale on the surface of the Earth, therefore it is convenient in that its value is equal to the weight of a body weighing 1 kg, so it is easy for a person to imagine, for example, what a force of 5 kgf is.
Kilogram-force (Russian designation: kgf or kg; international: kgf or kg F ) - unit of force in the MKGSS system of units ( M etr - TO silt G ramm- WITH ila - WITH second).
Technical atmosphere (at, at), kgf/cm 2
The technical atmosphere (Russian designation: at; international: at) is equal to the pressure produced by a force of 1 kgf, uniformly distributed over a flat surface perpendicular to it with an area of 1 cm 2. Thus,
1 at = 98,066.5 Pa
Physical atmosphere (atm, atm)
Normal, standard or physical atmosphere (Russian designation: atm; international: atm) - a non-systemic unit, equal to the pressure of a column of mercury 760 mm high on its horizontal base at a mercury density of 13,595.04 kg/m 3, at a temperature of 0°C and at normal acceleration of free fall is 9.80665 m/s 2.
1 atm = 760 mmHg.
According to the definition:
Millimeter of mercury
A millimeter of mercury (Russian designation: mm Hg; international: mm Hg) is a non-system unit of pressure measurement, sometimes called "torr" (Russian designation - Torr, international - Torr) in honor of Evangelista Torricelli.
1 mmHg ≈ 133.3223684 Pa
| atm sea level | 760 mmHg |
| 760 mmHg | 101 325 Pa |
| 1 mmHg | 101 325 / 760 ≈ 133.3223684 Pa |
| 1 mmHg |
13.5951 mm water column |
The origin of this unit is related to the method of measuring atmospheric pressure using a barometer, in which the pressure is balanced by a column of liquid. Mercury is often used as a liquid because it has a very high density (≈13,600 kg/m3) and low vapor pressure at room temperature.
Millimeters of mercury are used, for example, in vacuum technology, in weather reports and in measuring blood pressure.
The unit of measurement "inch of mercury" (symbol - inHg) is also used in the USA and Canada. 1 inHg = 3.386389 kPa at 0 °C.
Millimeter of water column
A millimeter of water column (Russian designation: mm water column, mm H 2 O; international: mm H 2 O) is a non-system unit of pressure measurement. Equal to the hydrostatic pressure of a column of water 1 mm high exerted on a flat base at a water temperature of 4 °C.
In the Russian Federation, it is approved for use as a non-systemic unit of pressure measurement without a time limit with the scope of use “all areas”.






