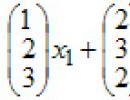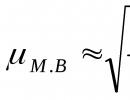2 methods of measuring physical quantities. Measurement of physical quantities: basic measurement methods. According to the way the results are expressed, they are distinguished
Lecture 3. MEASUREMENTS OF PHYSICAL QUANTITIES
3.1 Measurements of physical quantities and their classification
3.2 Principles, measurement methods
3.3. Measurement procedure
Measurements of physical quantities and their classification
The reliability of measurement information is the basis for analysis, forecasting, planning and production management in general, helps to increase the efficiency of accounting for raw materials, finished products and energy costs, as well as improve the quality of finished products.
Measurement- a set of operations performed to determine the quantitative value of a quantity;
Measurement of a physical quantity – a set of operations for the use of a technical device that stores a unit of physical quantity, ensuring that the relationship of the measured quantity with its unit is found and the value of this quantity is obtained.
Object of measurement– a real physical object, the properties of which are characterized by one or more measured PVs.
measuring technology– a set of technical means used to perform measurements.
The main consumer of measuring equipment is industry. here, measuring technology is an integral part of the technological process, as it is used to obtain information about technological regimes that determine the course of processes.
technological measurements– a set of measuring devices and measurement methods used in technological processes.
Object of measurement– a body (physical system, process, phenomenon, etc.), which is characterized by one or more measurable or measurable physical quantities.
Measurement quality is a set of properties that determine the compliance of means, method, methodology, measurement conditions and the state of measurement unity with the requirements of the measurement task.
Measurements are classified according to the following criteria:
3.1.1 According to the dependence of the measured value on time into static and dynamic ;
Static measurements– measurement of a physical quantity that, in accordance with the measurement task, is accepted as constant throughout the measurement time (for example, measuring the size of a part at normal temperature).
Dynamic measurements– measurements of a physical quantity whose size changes over time (for example, measurement of the mass fraction of water in a product during the drying process).
3.1.2 By method of obtaining results into direct, indirect, cumulative, joint;
Direct measurement– a measurement in which the desired value of a physical quantity is found directly from experimental data. In the process of direct measurement, the measurement object is brought into interaction with the measuring instrument and, according to the readings of the latter, the value of the measured quantity is measured. Examples of direct measurements include measuring length with a ruler, mass with a scale, temperature with a glass thermometer and active acidity with a pH meter, etc.
Direct measurements include measurements of the vast majority of parameters of a chemical technological process.
Indirect measurement– a measurement in which the desired value of a quantity is found on the basis of a known relationship between this quantity and quantities obtained by direct measurement.
Indirect measurements are used in two cases:
· there is no measuring instrument for direct measurements;
· Direct measurements are not accurate enough.
When conducting chemical analyzes of the composition and properties of food substances, indirect measurements are widely used. An example of indirect measurements can be measurements of the density of a homogeneous body by its mass and volume; determination of the mass fraction of water in fish products by drying at a temperature of 105 O C, the essence of which is to dry the product to a constant mass and determine the mass fraction of water according to the formula:
where M 1 – weight of the weighing bottle with a sample before drying, g; M 2 – weight of the weighing bottle with a sample after drying, g; M is the mass of the sample.
Cumulative measurements – measurements of several homogeneous quantities, in which the required values of the quantities are found by solving a system of equations obtained by direct measurements of various combinations of these quantities (measurements in which the mass of individual weights of a set is found from the known mass of one of them and from the results of direct comparisons of the masses of various combinations of weights).
Joint measurements – simultaneous measurements of two or more different quantities to find the relationship between them (for example, simultaneous measurements of the increment in the length of a sample depending on changes in its temperature and determination of the linear expansion coefficient using the formula k= l/(l Dt)).
Joint measurements are practically no different from indirect ones.
3.1.3. By connection with the object into contact and non-contact , at which the sensitive element of the device is brought into contact or not brought into contact with the measurement object.
3.1.4. According to accuracy conditions into equal and unequal.
Equal precision measurements – a series of measurements of any quantity made by measuring instruments of equal accuracy under the same conditions.
Unequal measurements– a series of measurements of any quantity, performed by measuring instruments of different accuracy and under different conditions. For example, the mass fraction of water in dried fish was determined by two methods: drying at a temperature of 130 O C and on the HF device at a temperature of 150 O C, the permissible error in the first case is +1%, in the second – +0.5%.
3.1.5 By the number of measurements in a series of measurements single and multiple.
Single measurement– measurements performed once (measurement of a specific time using a clock).
Multiple measurement– a measurement of a physical quantity of the same size, the result of which is obtained from several successive measurements, i.e. consisting of a series of single measurements. Typically, multiple measurements are those that are made more than three times. The arithmetic mean of individual measurements is usually taken as the result of multiple measurements.
3.1.6. According to metrological purpose for technical, metrological;
Technical Dimension– a measurement performed using a working measuring instrument for the purpose of monitoring and managing scientific experiments, monitoring product parameters, etc. (measuring temperature in a smoking oven, determining the mass fraction of fat in fish).
Metrological measurement– a measurement made using a standard and standard measuring instruments for the purpose of introducing a new unit of physical quantity or transferring its size to working measuring instruments.
3.1.7 By expressing the measurement result into absolute and relative;
Absolute measurement– a measurement based on direct measurements of one or more basic quantities and on the use of physical constants. For example, the measurement of gravity is based on the measurement of the basic quantity - mass (m) and the use of the physical constant g: F = mg.
Relative dimension– a measurement made with the aim of obtaining the ratio of a quantity to a quantity of the same name, which plays the role of a unit, or measuring a quantity in relation to a quantity of the same name, taken as the initial one. For example, measuring relative air humidity.
3.1.8. Based on existing sets of measured values on electrical ( current, voltage, power) , mechanical ( mass, number of products, effort); , thermal power(temperature, pressure); , physical(density, viscosity, turbidity); chemical(composition, chemical properties, concentration) , radio engineering etc.
Analysis of the state of measurements in the food industry made it possible to establish the qualitative and quantitative composition of the fleet of measuring equipment, which is characterized by the following ratio (%):
– thermal measurements – 50.7;
– mechanical measurements – 30.4;
– electric power – 12.1;
– physical and chemical measurements – 6.2;
– time and frequency measurements – 0.6.
Principles and methods of measurement
Measuring principle– a physical phenomenon or effect underlying measurements. For example, measuring temperature with a liquid thermometer is based on the increase in volume of liquid as temperature increases.
Measurement methodth- a technique or set of techniques for comparing a measured physical quantity with its unit in accordance with the implemented measurement principles.
The classification of measurement methods is presented in Fig. 3.1.

Figure 3.1. Classification of measurement methods
Direct assessment method– a measurement method in which the value of the measured quantity is determined directly from the reading device of a direct-acting measuring device (with reading on a scale or on a vernier scale - an auxiliary scale on which fractions of division of the main scale are counted). For example, counting by a clock or a ruler.
Comparison method with measure– a measurement method in which the measured value is compared with the value reproduced by the measure.
Measure– SI designed to reproduce PV of a given size
The comparison method happens zero, differential, substitution.
Null method– a type of differential method in which the resulting effect of the influence of quantities on a comparison device is brought to zero (cup scales). In this case, the value of the measured quantity is equal to the value that the measure reproduces.
At differential method the measured value x is compared directly or indirectly with the value x and a reproducible measure. The value of x is judged by the difference Δx = x – x m measured by the device in the simultaneously measured values x and xm and by the known value xm reproduced by the measure. Then
x = x m + Δx
Substitution method- a method in which the desired quantity is replaced by a measure with a known value.
Depending on the contact with the measured value, methods are divided into contact and non-contact , at which the sensitive element of the device is brought into contact or not brought into contact with the measurement object. An example of a contact measurement is measuring the temperature of a product with a thermometer, and a non-contact measurement is measuring the temperature in a blast furnace with a pyrometer.
Depending on the principle underlying the measurement, methods are divided into physical, chemical, physicochemical, microbiological, biological .
Physical method– the method is based on recording an analytical signal that records a certain property as a result of a physical process.
Using the physical method, the physical properties of hydrobionts (mass, length, color) and many technological process control parameters (temperature, pressure, time, etc.) are determined. When conducting research, various measuring instruments are used. This method is the most objective and progressive.
Advantages – speed of determination, accuracy of results
Disadvantages - the inability to determine many indicators, mainly analytical
Chemical method– is based on recording an analytical signal arising as a result of a chemical reaction, used to assess the composition and properties of a product. For example: titrometry (determination of salinity, gravimetry - determination of sulfate content in table salt).
Advantages: most accurate and objective.
Disadvantages: duration of analysis, requires preparation of reagents, large amount of glassware.
Physico-chemical method– is based on recording a signal that arises as a result of a chemical reaction, but which is also recorded in the form of a measurement of some physical property. Is currently the most progressive. Physico-chemical methods are divided into:
ABOUT optical methods– the connection between the optical properties of the system and its composition is used.
- calorimetric If - based on measuring the absorption of electromagnetic energy in a narrow range of light wavelengths (determining the amount of phenols, vitamin content, etc.).
- refractometric – based on measuring the refractive index of a solution (determination of dry matter content in a tomato).
- potentialometric– based on determining the equilibrium potential (measuring EMF) and finding the relationship between its value and the potential-determining component of the solution (Determination of pH of a solution)
- polarographic– based on determining the dependence of the current on the increase in voltage on the electrode of a cell immersed in a solution (determination of heavy metals)
- conductometric– based on determining the electrical conductivity of electrolyte solutions (determination of heavy metals, concentration of surface salt in solution).
- combined methods-based on the separation of complex mixtures into individual components and their quantitative determination, there are: chromatographic (thin-layer - determination of fatty acid composition; gas-liquid - determination of amino acid composition, pesticides, adsorption, ion exchange).
The measurement of physical quantities consists of comparing a quantity with a homogeneous quantity taken as a unit. In metrology, the term “measurement” is used, which means finding the value of a physical quantity experimentally using special technical means.
Measurements performed using special technical means are called instrumental. The simplest example of such measurements is determining the size of a part using a ruler with divisions, that is, comparing the size of the part with the unit of length stored by the ruler.
A derivative of the term "measurement" is the term "to measure", widely used in practice. There are terms “measure”, “measure”, “measure”, but their use in metrology is unacceptable.
To streamline measurement activities, measurements are classified according to the following criteria:
General methods of obtaining results - direct, indirect, compatible, cumulative;
Number of measurements in a series – single and multiple;
Metrological purposes – technical, metrological;
Characteristics of accuracy - equal and unequal;
Relation to changes in the measured value – statistical and dynamic;
Expression of measurement results – absolute and relative;
Direct measurements are measurements in which the desired value of a quantity is found directly from experimental data (measurements of mass on scales, temperature of thermometers, length using linear measures). In direct measurements, the object of study is brought into interaction with measuring instruments and, according to the readings of the latter, the value of the measured quantity is counted. Sometimes the instrument readings are multiplied by a coefficient, appropriate corrections are introduced, etc. These measurements can be written in the form of an equation: X = C X P,
where X is the value of the measured quantity in units accepted for it;
C – the price of a scale division or a single reading of a digital reading device in units of the measured value;
Х П – counting according to the indicator device in scale divisions.
Indirect measurements are measurements in which the desired value is found on the basis of a known relationship between this value and values obtained by direct measurements (determining the density of a homogeneous body by its mass and geometric dimensions, the electrical resistivity of a conductor by its resistance, length and cross-sectional area). In general, this dependence can be represented as a function X = (X1,X2,....,Xn), in which the value of the arguments X1, X2, ....,Xn is found as a result of direct, and sometimes indirect, joint or cumulative measurements .
For example, the density of a homogeneous solid body ρ is found as the ratio of mass m to its volume V, and the mass and volume of the body are measured directly: ρ=m/V.
To increase the accuracy of measurements of density ρ, measurements of mass m and volume V are carried out repeatedly. In this case, the body density
ρ = m/V, m is the result of measuring body mass, m = 1/n Σ m i;
V=ΣVi/n - the result of measuring the volume of the body Π.
Cumulative measurements - measurements of several homogeneous quantities, in which the desired value of the quantities is found by solving a system of equations obtained by direct measurements of various combinations of these quantities (measurements in which the mass of individual weights of a set is found from the known mass of one of them and from the results of direct comparisons of the masses of various combinations of weights ).
Joint measurements are simultaneous measurements of two or more opposite quantities to find the relationship between them (simultaneous measurements of the increment in the length of a sample depending on changes in its temperature and determination of the linear expansion coefficient).
Joint and cumulative measurements are very close in their methods of finding the desired values of the measured quantities. The difference is that with cumulative measurements, several quantities of the same name are simultaneously measured, and with joint measurements, they measure different quantities. The values of the measured quantities x1, ..., xn are determined on the basis of cumulative equations;
F1 (X1, ..., Xm, X11, ... , X1n);
F2 (X1, ..., Xm, X21, ... , X1n);
Fn (X1, ..., Xm, Xk1, ... , Xkn),
where X11, X21, ……………..Xk n are quantities measured by direct methods.
Joint measurements are based on well-known equations that reflect the connections existing in nature between the properties of objects, i.e. between quantities.
Absolute measurements are measurements based on direct measurements of one or more basic quantities and the use of physical constants.
Relative measurements - obtaining the ratio of a quantity to a quantity of the same name, which plays the role of a unit, or a change in a quantity in relation to a quantity of the same name, taken as the initial one.
Single measurements - a measurement performed once (measurement of a specific time using a clock).
Multiple measurements are measurements of the same physical quantity, the result of which is obtained from several successive measurements. Typically, multiple measurements are those that are made more than three times.
Technical measurements - measurements performed using working measuring instruments for the purpose of monitoring and managing scientific experiments, monitoring product parameters, etc. (measurement of air pressure in a car chamber).
Metrological measurements are measurements using standards and reference measuring instruments for the purpose of innovating units of physical quantities or transferring their sizes to working measuring instruments.
Equal-precision measurements are a series of measurements of any quantity made by measuring instruments of equal accuracy under the same conditions.
Non-equivalent measurements are a series of measurements of any quantity, performed with different accuracy with measuring instruments and under different conditions.
Static measurements are measurements of a physical quantity that, in accordance with a specific measurement task, is accepted as unchanged throughout the measurement time (measuring the size of a part at normal temperature).
Dynamic measurements are measurements of a physical quantity whose size changes over time (measuring the distance to ground level from a descending aircraft).
Measuring instruments
Measuring instruments are technical means used in measurements and having standardized metrological properties. The correct determination of the value of the measured quantity in the process of its measurement depends on the measuring instruments. Measuring instruments include: measures: measuring instruments, measuring installations, measuring systems.
A measure is a measuring instrument designed to reproduce a physical quantity of a given size (a weight is a measure of mass, a generator is a measure of the frequency of electrical oscillations). Measures, in turn, are divided into single-valued and multi-valued.
An unambiguous measure that reproduces a physical quantity of one size (plane-parallel gauge block, normal element, constant capacitance capacitor),
a multi-valued measure that reproduces a number of physical quantities of the same name of various sizes (ruler: in millimeter divisions, variable capacitor).
A set of measures is a specially selected set of measures used not only individually, but also in various combinations for the purpose of reproducing a number of quantities of the same name of various sizes (a set of weights, a set of plane-parallel gauge blocks).
A measuring device is a measuring instrument designed to generate a signal of measuring information in a form accessible to direct perception by an observer. The measurement results are produced by the reading devices of the instruments, which can be scale, digital and recording.
Scale reading devices consist of a scale, which is a set of marks and numbers depicting a series of sequential values of the measured quantity, and a pointer (arrow, electron beam, etc.) associated with the moving system of the device.
Scale marks with numerical values represented are called numeric scale marks. The main characteristics of the scale are the length of the scale division, expressed by the distance between the axes of two adjacent scale lines, and the value of the scale division, representing the value of the measured quantity, causing the pointer to move one division.
It is also customary to distinguish the following concepts: measurement range and reading range.
The measurement range is part of the reading range for which the limits of permissible errors of measuring instruments are normalized. The smallest and largest values of the measurement range are called the lower and upper limits of measurements, respectively.
The value of a quantity, determined by the reading device of a measuring instrument and expressed in the accepted units of this quantity, is called the reading of the measuring instrument.
The measured value is determined either by multiplying the number of scale divisions by the value of the scale division or by multiplying the numerical value read on the scale by the scale constant.
Currently, either mechanical or light-based digital reading devices are widely used.
Recording and reading devices consist of a writing or printing mechanism and a tape. The simplest writing device is a pen filled with ink, recording the measurement result on a paper tape. In more complex devices, the measurement result can be recorded by a light or electron beam, the movement of which depends on the values of the measured quantities.
Metrology, standardization and certification
General questions of the fundamentals of metrology and measuring technology
In practical life, people deal with measurements everywhere. At every step there are measurements of such quantities as length, volume, weight, time, etc.
Measurements are one of the most important ways for humans to understand nature. They provide a quantitative description of the world around us, revealing to humans the patterns operating in nature. All branches of technology could not exist without a comprehensive measurement system that determines all technological processes, their control and management, as well as the properties and quality of products.
The branch of science that studies measurements is metrology. The word "metrology" is formed from two Greek words: metron - measure and logos - doctrine. The literal translation of the word “metrology” is the study of measures. For a long time, metrology remained mainly a descriptive science about various measures and the relationships between them. Since the end of the 19th century, thanks to the progress of the physical sciences, metrology has received significant development. A major role in the development of modern metrology as one of the sciences of the physical cycle was played by D. I. Mendeleev, who led domestic metrology in the period 1892 - 1907.
In accordance with GOST 16263-70 “Metrology. Terms and Definitions": metrology is the science of measurements, methods and means of ensuring their unity and ways of achieving the required accuracy.
Unity of measurements- a state of measurements in which their results are expressed in legal units and measurement errors are known with a given probability. Unity of measurements is necessary in order to be able to compare the results of measurements taken in different places, at different times, using different methods and measuring instruments.
Accuracy of measurements characterized by the closeness of their results to the true value of the measured quantity. Accuracy is the reciprocal of errors(discussed below).
Measuring technology is a practical, applied area of metrology.
The measurable quantities with which metrology deals are physical quantities, i.e. quantities included in the equations of experimental sciences (physics, chemistry, etc.) involved in understanding the world empirical(T.
e. experimentally) by way.Metrology penetrates into all sciences and disciplines dealing with measurements, and is a single science for them.
The basic concepts that metrology operates on are the following:
Physical quantity;
Unit of physical quantity;
System of units of physical quantities;
Size of a unit of physical quantity (transfer of the size of a unit of physical quantity);
Instruments for measuring physical quantities;
Exemplary measuring instrument;
Working measuring instrument;
Measurement of a physical quantity;
Measurement method;
Measurement result;
Measurement error;
Metrological service;
Metrological support, etc.
Let's define some basic concepts:
Physical quantity– a characteristic of one of the properties of a physical object (phenomenon or process), common in qualitative terms for many physical objects, but quantitatively individual for each object (i.e., the value of a physical quantity can be for one object a certain number of times more or less than for the other). For example: length, time, electric current.
Unit of physical quantity– a physical quantity of a fixed size, which is conventionally assigned a numerical value equal to 1, and used for the quantitative expression of homogeneous physical quantities. For example: 1 m is a unit of length, 1 s is a unit of time, 1A is a unit of electric current.
System of units of physical quantities– a set of basic and derived units of physical quantities, formed in accordance with accepted principles for a given system of physical quantities. For example: International System of Units (SI), adopted in 1960.
In the system of units of physical quantities there are basic units of the system of units(in SI – meter, kilogram, second, ampere, kelvin). From the combination of basic units are formed derived units(speed - m/s, density - kg/m 3).
By adding installed prefixes to the basic units, multiple (for example, kilometer) or submultiple (for example, micrometer) units are formed.
Historically, the first system of units of physical quantities was the metric system of measures adopted in 1791 by the French National Assembly. It was not yet a system of units in the modern sense, but included units of length, area, volume, capacity and weight, which were based on two units: the meter and the kilogram.
In 1832, the German mathematician K. Gauss proposed a method for constructing a system of units as a set of basic and derivative ones. He constructed a system of units in which three arbitrary units independent of each other were taken as a basis - length, mass and time. All other units could be defined using these three. Gauss called such a system of units connected in a certain way with the three basic ones an absolute system. He took the millimeter, milligram and second as the basic units.
Subsequently, with the development of science and technology, a number of systems of units of physical quantities appeared, built on the principle proposed by Gauss, based on the metric system of measures, but differing from each other in basic units.
Let us consider the most important systems of units of physical quantities.
GHS system. The GHS system of units of physical quantities, in which the basic units are the centimeter as a unit of length, the gram as a unit of mass and the second as a unit of time, was established in 1881.
MKGSS system. The use of the kilogram as a unit of weight, and subsequently as a unit of force in general, led at the end of the 19th century to the formation of a system of units of physical quantities with three basic units: the meter - a unit of length, the kilogram-force - a unit of force and the second - a unit of time.
MCSA system. The foundations of this system were proposed in 1901 by the Italian scientist Giorgi. The basic units of the ISS system are the meter, kilogram, second and ampere.
The presence of a number of systems of units of physical quantities, as well as a significant number of non-system units, inconveniences associated with recalculation when moving from one system of units to another, required the unification of units of measurement. The growth of scientific, technical and economic ties between different countries necessitated such unification on an international scale.
A unified system of units of physical quantities was required, practically convenient and covering various areas of measurement. At the same time, it had to preserve the principle of coherence (equality to unity of the coefficient of proportionality in the equations of connection between physical quantities).
In 1954, the Tenth General Conference on Weights and Measures established six basic units (meter, kilogram, second, ampere, kelvin, candela + mole). The system, based on the six basic units approved in 1954, was called the International System of Units, abbreviated SI (SI - the initial letters of the French name Systeme International). A list of six basic, two additional and the first list of twenty-seven derivative units was approved, as well as prefixes for the formation of multiples and submultiples.
In the Russian Federation, the SI system is regulated by GOST 8.417-81.
Physical unit size– quantitative determination of a unit of physical quantity reproduced or stored by a measuring instrument. The size of the SI fundamental units is established by the definition of these units by the General Conference on Weights and Measures (GCPM). Thus, in accordance with the decision of the XIII CGPM, the unit of thermodynamic temperature, kelvin, is set equal to 1/273.16 of the thermodynamic temperature of the triple point of water.
Reproduction of units is carried out by national metrological laboratories using national standards. The difference between the size of the unit reproduced by the national standard and the size of the unit as defined by the CGPM is established during international comparisons of standards.
Unit size stored exemplary (OSI) or workers (RSI) measuring instruments, can be established in relation to the national primary standard. In this case, there may be several stages of comparison (through secondary standards and OSI).
Measurement of a physical quantity– a set of operations for the use of a technical means that stores a unit of physical quantity, consisting of comparison (explicitly or implicitly) of the measured quantity with its unit in order to obtain this quantity in the form most convenient for use.
Measuring principle– a physical phenomenon or effect underlying measurements using one or another type of measuring instrument.
Application of the Doppler effect to measure speed;
Application of the Hall effect to measure magnetic field induction;
Using gravity to measure mass by weighing.
Types of measurements
By the nature of the dependence of the measured quantity on time measurements are divided into:
static, in which the measured quantity remains constant over time;
dynamic, during which the measured quantity changes and is not constant over time.
Static measurements are, for example, measurements of body dimensions, constant pressure, electrical quantities in circuits with a steady state, dynamic - measurements of pulsating pressures, vibrations, electrical quantities under conditions of a transient process.
By method of obtaining measurement results they are divided into:
indirect;
cumulative;
joint.
Direct- these are measurements in which the desired value of a physical quantity is found directly from experimental data.
Direct measurements can be expressed by the formula , where is the desired value of the measured quantity, and is the value directly obtained from experimental data.In direct measurements, the measured quantity is subjected to experimental operations, which is compared with the measure directly or using measuring instruments calibrated in the required units. Examples of straight lines are measuring body length with a ruler, mass using scales, etc.
Indirect- these are measurements in which the desired quantity is determined on the basis of a known relationship between this quantity and quantities subjected to direct measurements, i.e. They measure not the actual quantity being determined, but others that are functionally related to it. The value of the measured quantity is found by calculating using the formula , where is the functional dependence, which is known in advance, and is the value of the quantities measured directly.
Examples of indirect measurements: determining the volume of a body by direct measurements of its geometric dimensions, finding the electrical resistivity of a conductor by its resistance, length and cross-sectional area.
Indirect measurements are widely used in cases where the desired quantity is impossible or too difficult to measure directly, or when direct measurement gives a less accurate result. Their role is especially great when measuring quantities that are inaccessible to direct experimental comparison, for example, dimensions of the astronomical or subatomic order.
Aggregate- these are measurements of several quantities of the same name made simultaneously, in which the desired quantity is determined by solving a system of equations obtained by direct measurements of various combinations of these quantities.
An example of cumulative measurements is the determination of the mass of individual weights in a set (calibration using the known mass of one of them and the results of direct comparisons of the masses of various combinations of weights).
Joint- these are measurements of two or several quantities of different names made simultaneously to find dependencies between them.
An example is the measurement of electrical resistance at 20 0 C and the temperature coefficients of a measuring resistor based on direct measurements of its resistance at different temperatures.
Measurement methods
Method of measurement is a method of experimentally determining the value of a physical quantity, i.e., a set of physical phenomena and measuring instruments used in measurements.

Direct assessment method consists in determining the value of a physical quantity using the reading device of a direct-acting measuring device. For example, measuring voltage with a voltmeter.
This method is the most common, but its accuracy depends on the accuracy of the measuring instrument.
Method of comparison with a measure - in this case, the measured value is compared with the value reproduced by the measure. The measurement accuracy may be higher than the accuracy of direct assessment.
There are the following types of comparison method with a measure:
Contrasting method, in which the measured and reproduced quantity simultaneously influence the comparison device, with the help of which the relationship between the quantities is established. Example: Measuring weight using a lever scale and a set of weights.
Differential method, in which the measuring device is affected by the difference between the measured value and the known value reproduced by the measure. In this case, the balancing of the measured value with a known one is not carried out completely. Example: Measuring DC voltage using a discrete voltage divider, a reference voltage source, and a voltmeter.
Null method, in which the resulting effect of the influence of both quantities on the comparison device is brought to zero, which is recorded by a highly sensitive device - a zero indicator. Example: Measuring the resistance of a resistor using a four-arm bridge, in which the voltage drop across a resistor of unknown resistance is balanced by the voltage drop across a resistor of known resistance.
Substitution method, in which the measured quantity and a known quantity are alternately connected to the input of the device, and the value of the measured quantity is estimated from two readings of the device, and then by selecting a known quantity, they are ensured that both readings coincide. With this method, high measurement accuracy can be achieved with a high precision measure of a known quantity and high sensitivity of the device. Example: accurate, precise measurement of a small voltage using a highly sensitive galvanometer, to which a source of unknown voltage is first connected and the deflection of the pointer is determined, and then using an adjustable source of known voltage, the same deflection of the pointer is achieved. In this case, the known voltage is equal to the unknown.
Match Method, in which the difference between the measured value and the value reproduced by the measure is measured using the coincidence of scale marks or periodic signals. Example: measuring the rotation speed of a part using a flashing strobe lamp: observing the position of the mark on the rotating part at the moments of the lamp flashes, the speed of the part is determined from the known frequency of the flashes and the displacement of the mark.
Factors influencing measurement results
In metrological practice, when carrying out measurements, it is necessary to take into account a number of factors that influence the measurement results. These are the object and subject of measurement, measurement method, measurement tool and measurement conditions.
Object of measurement must be free from foreign inclusions if the density of a substance is being measured, free from the influence of external interference (natural processes, industrial interference, etc.). The object itself should not have internal interference (the operation of the measurement object itself).
Subject of measurement, that is, the operator introduces into the result a “personal” moment of measurement, an element of subjectivity. It depends on the qualifications of the operator, sanitary and hygienic working conditions, the psychophysiological state of the subject, and taking into account ergonomic requirements.
Method of measurement. Very often, measuring the same quantity of a constant size using different methods gives different results, and each of them has its own disadvantages and advantages. The art of the operator is to eliminate or take into account by appropriate means factors that distort the results. If the measurement cannot be performed in such a way as to exclude or compensate for any factor influencing the result, then in a number of cases an appropriate amendment is made to the latter.
Impact of SI The measured quantity in many cases manifests itself as a disturbing factor, for example, internal noise of measuring electronic amplifiers.
Another factor is the inertia of SI. Some SIs give consistently high or low readings, which may be the result of a manufacturing defect.
Measurement conditions influencing factors include ambient temperature, humidity, atmospheric pressure, network voltage, etc.
Taking these factors into account involves eliminating errors and making corrections to the measured values.
Measurement methods are determined by the type of measured quantities, their sizes, the required accuracy of the result, the required speed of the measurement process and other data.
There are many measurement methods, and as science and technology develop, their number is increasing.
According to the method of obtaining the numerical value of the measured value, all measurements are divided into three main types: direct, indirect and cumulative.
Direct are called measurements in which the desired value of a quantity is found directly from experimental data (for example, measuring mass on a dial or equal-arm scale, temperature - with a thermometer, length - using linear measures).
Indirect are called measurements in which the desired value of a quantity is found on the basis of a known relationship between this quantity and quantities subjected to direct measurements (for example, the density of a homogeneous body based on its mass and geometric dimensions; determination of electrical resistance from the results of measuring voltage drop and current).
Cumulative are called measurements in which several quantities of the same name are simultaneously measured, and the desired value of the quantities is found by solving a system of equations obtained from direct measurements of various combinations of these quantities (for example, measurements in which the masses of individual weights of a set are determined from the known mass of one of them and from the results of direct comparisons of masses of different combinations of weights).
It was said earlier that in practice direct measurements are most widespread due to their simplicity and speed of execution. Let us give a brief description of direct measurements.
Direct measurements of quantities can be made using the following methods:
1) Direct assessment method- the value of the quantity is determined directly from the reading device of the measuring device (measurement of pressure - with a spring pressure gauge, mass - with dial scales, electric current - with an ammeter).
2) Comparison method with measure - the measured value is compared with the value reproduced by the measure (measuring mass with lever scales balanced with weights).
3) Differential method- a method of comparison with a measure, in which the measuring device is affected by the difference between the measured value and the known value reproduced by the measure (measurements performed when checking length measures by comparison with a standard measure on a comparator).
4) Null method- method of comparison with a measure, when the resulting effect of the influence of quantities on a comparison device is brought to zero (measurement of electrical resistance with a bridge with its complete balancing).
5) Match method- a method of comparison with a measure, in which the difference between the measured value and the value reproduced by the measure is measured using the coincidence of scale marks or periodic signals (length measurement using a vernier caliper, when the coincidence of marks on the caliper and vernier scales is observed).
6) Substitution method - method of comparison with a measure, when the measured value is replaced by a known value, reproducible by the measure (weighing with alternately placing the measured mass and weights on the same pan of scales).
Ministry of Education and Science of the Russian Federation Federal State Budgetary Educational Institution of Higher Professional Education žKuzbass State Technical University named after. T. F. Gorbacheva¤
Department of metal-cutting machines and tools
METHODS AND TOOLS FOR MEASUREMENT OF PHYSICAL QUANTITIES
Guidelines for performing laboratory work in the disciplines žMetrology, standardization and certification¤, žMetrology and certification¤
for students of directions 221400, 280700, 130400.65 full-time study
Compiled by D. M. Dubinkin
Approved at a meeting of the department Minutes No. 2 of 10.20.2011
An electronic copy is in the KuzSTU library
KEMEROVO 2011
1. PURPOSE OF THE WORK
The purpose of laboratory work is to study physical quantities, principles and methods of measuring physical quantities, as well as gaining knowledge about measuring instruments.
2. BASIC PROVISIONS
Metrology is the science of measurements, methods and means of ensuring their unity and ways of achieving the required accuracy.
Metrology studies:
– methods and means for accounting for products according to the following indicators: length, weight, volume, consumption and power;
– measurements of physical quantities (PV) and technical parameters, as well as the properties and composition of substances;
– measurements for control and regulation of technological processes.
There are several main areas of metrology:
– general measurement theory;
– PV unit systems;
– methods and means of measurement;
– methods for determining measurement accuracy;
– the basis for ensuring the uniformity of measurements, as well as the basis for the uniformity of measuring instruments;
– standards and exemplary measuring instruments;
– methods of transferring unit sizes from samples of measuring instruments and from standards to working measuring instruments.
The following metrology objects are distinguished:
– PV units;
– measuring instruments (MI);
– methods and techniques of measurements.
Modern metrology includes three components (Fig. 1): theoretical (fundamental, scientific), applied (practical) and legal metrology.
Theoretical metrology deals with issues of fundamental research, the creation of a system of units of measurement, physical constants, and the development of new measurement methods.
Metrology
Methods, means and methods of measurement
Theory of uniformity of measurements
1. PV units
2. Standards
3. Theory of transmission units of PV
Theory of measurement accuracy
Definition
errors
measurements
Rice. 1. Block diagram of metrology
Applied metrology deals with practical application in various fields of activity of the results of theoretical research within the framework of metrology and the provisions of legal metrology.
Legal metrology includes a set of interdependent rules and norms, which are binding and under the control of the state, on the use of PV units, standards, methods and measuring instruments, aimed at ensuring the uniformity of measurements in the interests of society.
3. PHYSICAL QUANTITIES
Physical quantity(PV) – one of the properties of a physical object (physical system, phenomenon or process), common in some way
qualitatively for many physical objects, but quantitatively individual for each of them.
A quantity is a property of something that can be distinguished from other properties and assessed in one way or another, including for the quantitative description of various properties of processes and physical bodies. A quantity does not exist on its own; it exists only insofar as there is an object with properties expressed by a given quantity.
Values can be divided into real and ideal. Ideal quantities mainly relate to mathematics and are a generalization (model) of specific real concepts. Real quantities are divided, in turn, into physical and non-physical. In the general case, PV can be defined as a quantity characteristic of material objects (processes, phenomena). Non-physical quantities include quantities inherent in social (non-physical) sciences - philosophy, sociology, economics, etc.
It is advisable to divide PV into measured and assessed. Measured EF can be expressed quantitatively in the form of a certain number of established units of measurement. The possibility of introducing and using the latter is an important distinguishing feature of measured EF. PVs for which, for one reason or another, a unit of measurement cannot be entered, can only be estimated. Values are assessed using scales.
Non-physical quantities, for which a unit of measurement cannot in principle be introduced, can only be estimated.
The use of the short form of the term žmagnitude¤ instead of the term žPV¤ is permissible only when it is clear from the context that we are talking about PV, and not about mathematical one.
The term “quantity” should not be used to express only the quantitative side of the property under consideration. For example, you cannot say or write “mass”, “area”, “current”, etc., since these characteristics (mass, area, current) are themselves quantities. In these cases, the terms “size of quantity” or “value of quantity” should be used.
Measurable EF – EF to be measured, measured or measured in accordance with the main purpose of the measurement task.
The size of the PV is the quantitative determination of the PV inherent in a specific material object, system, phenomenon or process.
The PV value is an expression of the PV size in the form of a certain number of units accepted for it.
The meaning of the magnitude should not be confused with the size. The size of the PV of a given object really exists and regardless of whether we know it or not, whether we express it in any units or not. The value of the PV appears only after the size of the value of a given object is expressed using some unit.
Numerical value of PV– an abstract number included in the value of a quantity.
True value of PV– PV value, which ideally characterizes the corresponding PV in qualitative and quantitative terms.
The true meaning of PV can be correlated with the concept of absolute truth. It can only be obtained as a result of an endless process of measurements with endless improvement of methods and measuring instruments (MI). For each level of development of measurement technology, we can only know the actual value of the PV, which is used instead of the true value of the PV. The concept of the true value of a physical quantity is necessary as a theoretical basis for the development of measurement theory, in particular, when revealing the concept of “measurement error”.
Actual PV value – PV value obtained experimentally and so close to the true value that it can be used instead of it in the given measurement task. The actual value of the PV is usually taken to be the arithmetic mean of a series of value values obtained with equal-precision measurements, or the weighted arithmetic mean with unequal-precision measurements.
Physical parameter– PV, considered when measuring a given PV as auxiliary. When assessing product quality, the expression measured parameters is often used. Here, parameters usually mean PV, which usually best reflects the quality of products or processes.
Influencing PV - PV that influences the size of the measured quantity, the measurement of which is not provided for in the data.
a special measuring instrument (MI), but influencing the results of measurements of the PV for which the SI is intended.
A PV system is a set of PVs formed in accordance with accepted principles, when some quantities are taken as independent, while others are defined as functions of independent quantities.
In the name of the system of quantities, symbols of quantities taken as basic are used. So the system of quantities of mechanics, in which
V length taken as basic ( L), mass (M) and time (T), is called the LMT system.
The system of basic quantities corresponding to the International System of Units (SI) is denoted by the symbols LMTIΘNJ, denoting, respectively, the symbols of the basic quantities - length (L), mass (M), time (T), electric current (I), temperature (Θ), quantity substance (N) and luminous intensity (J).
Main PV – PV included in the system and conditionally accepted
V as independent of other quantities of this system. Derivative of PV - PV included in the system and determined
through the basic quantities of this system.
The dimension of a PV is an expression in the form of a power monomial, composed of products of symbols of the main PVs in various powers and reflecting the connection of a given PV with the PVs adopted
V given system of quantities as basic ones with a proportionality coefficient equal to 1.
The powers of the symbols of the basic quantities included in the monomial are
V depending on the connection of the considered PV with the main ones, they can be integer, fractional, positive and negative. The concept of dimension extends to basic quantities. The dimension of the main quantity in relation to itself is equal to one, that is, the formula for the dimension of the main quantity coincides with its symbol.
IN in accordance with ISO 31/0 standard dimensions of quantities
should be denoted by dim. For example, the dimension of speed dim ν = LT - 1.
PV dimension indicator– exponent to which the dimension of the main PV is raised, which is included in the dimension of the derivative PV. The dimension indicator of the main PV in relation to itself is equal to one.
Dimensional PV – PV, in the dimension of which at least one of the main PVs is raised to a power not equal to zero. For example, force (F) in the LMTIΘNJ system is a dimensional quantity.
Dimensionless PV - PV, the dimension in which the main PVs are included to a power equal to zero. The PV in one system of quantities can be dimensional, and in another system it can be dimensionless. For example, the electric constant in the electrostatic system is a dimensionless quantity, but in the SI system of quantities it has a dimension.
Equation of connection between quantities – an equation reflecting the relationship between quantities determined by the laws of nature, in which the letter symbols are understood as PV. The equation for the relationship between quantities in a specific measurement task is often called the measurement equation.
The genus of the PV is the qualitative certainty of the PV. For example: the length and diameter of a part are homogeneous quantities; the length and mass of the part are non-uniform quantities.
Additive PV – PV, different values of which can be summed, multiplied by a numerical coefficient, or divided by each other. Additive quantities include length, mass, force, pressure, time, speed, etc.
Non-additive PV – PV for which summing, multiplying by a numerical coefficient or dividing its values by each other has no physical meaning (for example, thermodynamic temperature, material hardness).
4. UNITS OF PHYSICAL QUANTITIES
Unit of measurement PV– PV of a fixed size, which is conditionally assigned a numerical value equal to 1, and used for the quantitative expression of PV similar to it.
In practice, the concept of legalized units is widely used - a system of units and (or) individual units established for use in the country in accordance with legislative acts.
PV unit system– a set of basic and derived units formed in accordance with the principles for a given system of physical quantities.
Basic unit of PV– unit of the main PV in a given system of units.
Derived unit of the PV unit system – a unit of the derivative of the PV system of units, formed in accordance with the equation connecting it with the basic units or with the basic and already defined derivatives. For example: 1 m/s – a unit of speed formed from the basic SI units – meters and seconds; 1 N is a unit of force derived from the SI base units of the kilogram, meter and second.
GOST 8.417 establishes seven main PVs (Table 1) with the help of which the entire variety of PV derivatives is created and a description of any properties of physical objects and phenomena is provided.
Table 1 |
|||||
Major units of the international system (SI) |
|||||
Magnitude |
|||||
Name |
Name |
Designation |
|||
folk |
|||||
Basic units |
|||||
kilogram |
|||||
Electrical power |
|||||
ski current |
|||||
Thermodynamic |
|||||
sky temperature |
|||||
Quantity |
|||||
substances |
|||||
The power of light |
|||||
Some derived units |
|||||
square |
|||||
cubic |
|||||
Speed |
L T -1 |
||||
Meter is the length of the path traveled by light in a vacuum during a time interval of 1/299,792,458 s.
A kilogram is a unit of mass equal to the mass of the international prototype of the kilogram.
A second is a time equal to 9,192,631,770 periods of radiation corresponding to the transition between two hyperfine levels of the ground state of the cesium-133 atom, in the absence of disturbance from external fields.
Ampere is the strength of an unchanging current, which, when passing through two parallel conductors of infinite length and negligibly small cross-section, located in a vacuum at a distance of 1 m from each other, would cause on each section of the conductor 1 m long an interaction force equal to 2 10- 7 N.
Kelvin is a unit of thermodynamic temperature equal to 1/273.16 of the thermodynamic temperature of the triple point of water.
Mole is the amount of substance containing the same number of structural elements as there are atoms in carbon 12 weighing 0.012 kg. Structural elements can be atoms, molecules, ions and other particles.
Candela is the luminous intensity in a given direction of a source emitting monochromatic radiation with a frequency of 540·1012 Hz, the luminous energy intensity in this direction is 1/683 W/sr.
There are the following derived units of the PV unit system:
– formed from basic units (for example, the unit of area is a square meter);
– having special names and designations (for example, the unit of frequency is hertz).
When constructing a PV system, a sequence of defining equations is selected in which each subsequent equation contains only one new derived quantity, which makes it possible to express this quantity through a set of previously determined quantities, and, ultimately, through the basic quantities of the system of quantities.
To find the dimension of the derivative of the PV in a certain system of quantities, it is necessary to substitute their dimensions into the right side of the defining equation of this quantity instead of the designations of quantities (see Table 1). So, for example, putting in the determinant
equation for the speed of uniform motion ν = ds / dt instead of ds
dimension of length L and instead of dt dimension of time T, we get: dim ν = L / T = LT -1.
Substituting into the defining equation of acceleration a = dν / dt instead of dt the dimension of time T and instead of dν the dimension of speed found above, we obtain: dim a = LT -1 / T = LT -2.
Knowing the dimension of acceleration according to the defining equation of force F = ma, we obtain: dim F = M · LT -2 =LMT -2.
Knowing the dimension of force, you can find the dimension of work, then the dimension of power, etc.
PV system unit– PV unit included in the accepted system of units. The SI basic, derivative, multiple and submultiple units are systemic. For example: 1 m; 1 m/s; 1 km; 1 nm.
Non-system unit of PV– a unit of PV that is not included in the accepted system of units (for example, millimeter of mercury - mm Hg, bar - bar). Non-system units (in relation to SI units) are divided into four groups:
– accepted on a par with SI units;
– approved for use in special areas;
– temporarily admitted;
– obsolete (invalid).
Coherent derived unit of PV – a derivative unit of the PV, related to other units of the system of units by an equation in which the numerical coefficient is taken equal to 1.
Coherent system of PV units – a system of PV units, consisting of basic units and coherent derivative units. Multiples and submultiples of system units are not included in the coherent system.
FV multiple unit– a unit of physical activity, an integer number of times larger than a systemic or non-systemic unit. For example: unit of length 1 km = 103 m, i.e. a multiple of a meter; frequency unit 1 MHz (megahertz) = 106 Hz, multiple of hertz; unit of radionuclide activity 1 MBq (megabecquerel) = 106 Bq, a multiple of becquerel.
FV lobe unit– a unit of physical activity, an integer number of times smaller than a systemic or non-systemic unit. For example: unit of length 1 nm (nanometer) = 10-9 m; time unit 1 µs = 10-6 s are submultiples of a meter and a second, respectively.