Deoxyribose is a monosaccharide that plays an important biological role. Monosaccharides: ribose, deoxyribose, glucose, fructose. The concept of spatial isomers of carbohydrates. Cyclic forms of monosaccharides Difference between ribose and deoxyribose
Composed of 5 carbon atoms (pentose), which is formed from ribose when it loses one oxygen atom. The empirical chemical formula for deoxyribose is C5H10O4, and due to the loss of an oxygen atom it does not agree with the general formula for monosaccharides (CH2O)n, where n is an integer.
Physical and chemical properties
The linear formula of deoxyribose can be represented as follows: H-(C=O)-(CH2)-(CHOH) 3 -H. However, it also exists in the form of a closed ring of carbon atoms.
Deoxyribose is a colorless solid that is odorless and highly soluble in water. Its molecular weight is 134.13 g/mol, melting point 91 °C. It is obtained from ribose-5-phosphate due to the action of appropriate enzymes during a chemical reduction reaction.
Difference between ribose and deoxyribose
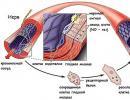
As already mentioned and as the name indicates, deoxyribose is a chemical compound whose atomic composition differs from that of ribose by only one oxygen atom. As shown in the figure below, deoxyribose does not have an OH hydroxyl group on the second carbon atom.
Deoxyribose is part of the chain while ribose is part of the acid).
It is interesting to note that the monosaccharides arabinose and ribose are stereoisomers, that is, they differ in spatial arrangement relative to the plane of the ring of the OH group near the 2nd carbon atom. Deoxyarabinose and deoxyribose are the same compound, but the second name is used because this molecule is obtained from ribose.
Deoxyribose and genetic information
Since deoxyribose is part of the DNA chain, it plays an important role - a source of genetic information; it consists of nucleotides, which include deoxyribose. Deoxyribose molecules link one nucleotide to another in the DNA chain through phosphate groups.

It has been established that the absence of the OH hydroxyl group in deoxyribose imparts mechanical flexibility to the entire DNA chain compared to RNA, which, in turn, allows the DNA molecule to form a double strand and be in a compact form inside the cell nucleus.
In addition, due to the flexibility of the bonds between nucleotides formed by deoxyribose molecules and phosphate groups, the DNA chain is much longer than RNA. This fact allows genetic information to be encoded at high density.
Chemical encyclopedia. - M.: Soviet Encyclopedia. Ed. I. L. Knunyants. 1988 .
See what "2-DEOXY-D-RIBOSE" is in other dictionaries:
2-deoxy-D-ribose
Deoxyribose 2-deoxy-D-ribose- Deoxyribose, 2 deoxy D ribose * deoxyribose, 2 deoxy D fishose * deoxyribose five-carbon sugar, which is a structural element of DNA (see). Monosaccharide from the group of deoxysugars. It is part of DNA and is in the furan form, where... ... Genetics. encyclopedic Dictionary
- (aminodeoxysugars), monosaccharides, in molecules instead of one or more. hydroxyl groups (except for hemiacetal in aldoses or hemiketal in ketoses) contain unsubstituted and substituted amino groups. A. also includes monosaccharides,... ... Chemical encyclopedia
Monosaccharides containing one or more in a molecule. hydrogen atoms instead of hydroxyl groups. According to IUPAC rules, the name of the D. must indicate abs. (D or L) and relative configurations, deoxy unit position and carbon chain length, e.g.... Chemical encyclopedia
- (sugars), a large group of polyhydroxycarbonyl compounds that are part of all living organisms; U. also includes many. derivatives obtained by chemical Modifications of these connections. by oxidation, reduction or introduction of decomposition. deputies... ... Chemical encyclopedia
2 deoxy B ribose, a monosaccharide from the group of deoxy sugars; is part of deoxyribonucleic acid (DNA) material carriers of heredity. It is found in DNA in the furanose form, the first carbon atom D. is associated with a nitrogenous base, and C3... ... Biological encyclopedic dictionary
Deoxyribonucleic acid (DNA)- a molecule consisting of a paired carbohydrate base (2 deoxy D ribose) and nucleotides located on it in a certain sequence (adenine, guanine, cytosine and thymine). The structure of the DNA molecule was discovered by D.D. Watson and F. Crick (1953),... ... Encyclopedic Dictionary of Psychology and Pedagogy
DEOXYRIBONUCLEIC ACID (DNA)- A large complex molecule consisting of four nucleotide bases (adenine, guanine, cytosine and thymine) and a carbohydrate base (2 deoxy D ribose). Nucleotide bases are arranged in pairs, oriented towards the center of the molecule in the form... ... Explanatory dictionary of psychology
2-deoksi-D-ribozė- statusas T sritis chemija apibrėžtis Aldopentozė, DNR struktūros sudedamoji dalis. formulė H(CHOH)₃CH₂CHO atitikmenys: engl. 2 deoxy D ribose rus. 2 deoxy D ribose... Chemijos terminų aiškinamasis žodynas
2-deoxy-D-ribose- 2 deoksi D ribozė statusas T sritis chemija apibrėžtis Aldopentozė, DNR struktūros sudedamoji dalis. formulė H(CHOH)₃CH₂CHO atitikmenys: engl. 2 deoxy D ribose rus. 2 deoxy D ribose... Chemijos terminų aiškinamasis žodynas
From monosaccharides, when hydroxyl groups are replaced by an amino group (-NH 2), amino sugars are formed. The most important amino sugars in the human body are glucosamine and galactosamine:
They are part of the complex carbohydrates of mucopolysaccharides, which perform protective and specific functions characteristic of mucus, the vitreous body of the eye, synovial fluid of joints, the blood coagulation system, etc.
Many functionally important substances are formed from glucose in the process of its oxidation or reduction: ascorbic acid, sorbitol alcohol, gluconic, glucuronic, sialic and other acids.
2.1.4. Ribose and deoxyribose
These carbohydrates are rarely found in free form. More often they are part of complex substances, i.e. used in the body in plastic processes. Thus, ribose is part of nucleotides (ATP, ADP, AMP) and RNA, as well as many coenzymes (NADP, NAD, FAD, FMN, CoA). Deoxyribose is part of DNA. In the body, ribose and deoxyribose (like other pentoses) are in a cyclic form.

2.1.5. Glyceraldehyde and dihydroxyacetone
They are formed in body tissues during the metabolism of glucose and fructose. Being isomers, these trioses are capable of interconversion:

In the tissues of the body, during the metabolism of carbohydrates and fats, phosphorus esters of glyceraldehyde and phosphodioxyacetone are formed. Phosphoglyceraldehyde is a high-energy substrate for biological oxidation. During its oxidation, ATP, pyruvic acid (PVA) and lactic acid (lactate) are formed.
Monosaccharides easily enter into chemical interactions, therefore they are rarely found in living organisms in a free state. Oligosaccharides are especially important derivatives of monosaccharides for the body.
2.2. Oligosaccharides
These are complex carbohydrates, built from a small number (from 2 to 10) of monosaccharide residues. If two monosaccharide residues are connected to each other by 1,4 or 1,2-glycosidic bonds, then disaccharides are formed. The main disaccharides are sucrose, maltose and lactose. Their molecular formula is C 12 H 22 O 12.
2.2.1. Sucrose
Sucrose- (cane or beet sugar) consists of a glucose and fructose residue connected by a 1,2-glycosidic bond, which is formed by the interaction of the hydroxyl group of the first carbon atom of glucose and the hydroxyl group of the second carbon atom of fructose.

Sucrose is the main component of table sugar. During the digestion process, under the influence of the enzyme sucrase, it is broken down into glucose and fructose.
2.2.2. Maltose
Maltose- (fruit sugar) consists of two glucose molecules connected by a 1,4-glycosidic bond:

A lot of maltose is found in malt extracts of cereals and sprouted grains. It is formed in the gastrointestinal tract during the hydrolysis of starch or glycogen. During digestion, it breaks down into two glucose molecules under the influence of the enzyme maltase.
2.2.3. Lactose
Lactose- (milk sugar) consists of glucose and galactose molecules, which are connected by a 1,4-glycosidic bond:

Lactose is synthesized in the mammary glands during lactation. In the human digestive system, lactose is broken down by lactase into glucose and galactose. The intake of lactose into the body with food promotes the development of lactic acid bacteria, which suppress the development of putrefactive processes. However, people with low activity of the lactase enzyme (the majority of the adult population of Europe, the East, Arab countries, India) develop intolerance to milk.
The disaccharides considered have a sweet taste. If the sweetness of sucrose is taken as 100, then the sweetness of lactose will be 16, maltose -30, glucose -70, fructose -170. In addition, they also have high nutritional value. Therefore, they are not recommended for people suffering from obesity and diabetes. They are replaced with artificial substances, such as saccharin, which have a sweet taste (saccharin sweetness -40000), but are not absorbed by the body.
Most carbohydrates in nature are found in the form of polysaccharides and are divided into two large groups - homo- and heteropolysaccharides.
§ 2. MONOSACHARIDES
Spatial isomerism
By their chemical nature, monosaccharides are aldehyde or keto alcohols. The simplest representative of monosaccharides, aldotriose, is glyceraldehyde (2,3-dihydroxypropanal).
Considering the structure of glyceraldehyde, one can notice that the given formula corresponds to two isomers that differ in spatial structure and are mirror images of each other:
Isomers that have the same molecular formulas but differ in the arrangement of atoms in space are called spatial, or stereoisomers. Two stereoisomers related to each other as an object and a mirror image that does not coincide with it are called enantiomers. This type of spatial isomerism is also called optical isomerism.
The existence of enantiomers in glyceraldehyde is due to the presence in its molecule chiral carbon atom, i.e. atom bonded to four different substituents. If there is more than one chiral center in a molecule, then the number of optical isomers will be determined by the formula 2 n, where n is the number of chiral centers. In this case, stereoisomers that are not enantiomers are called diastereomers.
To depict optical isomers on a plane, use Fischer projections. When constructing Fischer projections, it should be taken into account that atoms or groups of atoms lying on a horizontal line must be directed towards the observer, i.e. come out of the plane of the paper. Atoms or groups of atoms lying on a vertical line and, as a rule, making up the main chain, are directed away from the observer, i.e. go beyond the plane of the paper. For the isomers of glyceraldehyde we are considering, the construction of Fischer projections will proceed as follows:

Glyceraldehyde is accepted as the standard for the designation of optical isomers. To do this, one of its isomers was designated by the letter D, and the second by the letter L.
Pentoses and hexoses
As mentioned above, aldopentoses and aldohexoses are the most common in nature. Considering their structure, we can come to the conclusion that aldopentoses have 3 chiral centers (indicated by asterisks) and, therefore, consist of 8 (2 3) optical isomers. Aldohexoses have 4 chiral centers and 16 isomers:
Comparing the structure of the latter from the carbonyl group of the chiral center of the carbohydrate with the structure of D- and L-glyceraldehydes, all monosaccharides are divided into two groups: D- and L-series. The most important representatives of aldopentoses are D-ribose, D-deoxyribose, D-xylose, L-arabinose, aldohexoses - D-glucose and D-galactose, and ketohexoses - D-fructose. Fischer projections of the named monosaccharides and their natural sources are given below.
Monosaccharides exist not only in the form of open (linear) forms, which are given above, but also in the form of cycles. These two forms (linear and cyclic) are capable of spontaneously transforming into one another in aqueous solutions. The dynamic equilibrium between structural isomers is called tautomerism. The formation of cyclic forms of monosaccharides occurs as a result of the intramolecular addition of one of the hydroxyl groups to the carbonyl group. The most stable are five- and six-membered cycles. Therefore, when cyclic forms of carbohydrates are formed, furanose(five-membered) and pyranose(six-membered) cycles. Let us consider the formation of cyclic forms using the examples of glucose and ribose.
When cyclized, glucose forms predominantly a pyranose cycle. The pyranose cycle consists of 5 carbon atoms and 1 oxygen atom. When it is formed, the hydroxyl group of the fifth (C 5) carbon atom participates in the addition.
In place of the carbonyl group, a hydroxyl group appears, which is called glycosidic, and derivatives of the glycosidic group of carbohydrates – glycosides. Another spatial feature of cyclic forms is the formation of a new chiral center (C 1 atom). Two optical isomers arise, which are called anomers. The anomer in which the glycosidic group is located in the same way as the hydroxyl group, which determines the relationship of the monosaccharide to the D- or L-series, is designated by the letter, the other anomer by the letter. The structure of monosaccharides in cyclic form is often depicted in the form of Haworth's formulas. This image allows you to see the relative position of hydrogen atoms and hydroxyl groups relative to the plane of the ring.
Thus, in solution, glucose exists in the form of three forms that are in mobile equilibrium, the ratio between which is approximately: 0.025% - linear form, 36% - - and 64% - - form.
Ribose forms mainly five-membered furanose rings.
Chemical properties
The chemical properties of monosaccharides are determined by the presence of a carbonyl group and alcohol hydroxyls in their molecules. Let's look at some reactions of monosaccharides using glucose as an example.
Like a polyhydric alcohol, glycol, glucose solution dissolves the precipitate of copper (II) hydroxide to form a complex compound.
The aldehyde group upon reduction forms alcohols. When glucose is reduced, a hexahydric alcohol is formed sorbitol:
Sorbitol has a sweet taste and is used as a sugar substitute. Xylitol, a product of xylose reduction, is also used for the same purpose.
In oxidation reactions, depending on the nature of the oxidizing agent, monobasic (aldonic) or dibasic (glucaric) acids can be formed.
Most monosaccharides are reducing sugars. They are characterized by: the “silver mirror” reaction
and interaction with Fehling's liquid (reduction of blue Cu(OH) 2 to yellow CuOH and then orange Cu 2 O).
The glycosidic group of cyclic forms of monosaccharides has increased reactivity. Thus, when interacting with alcohols, ethers are formed - glycosides. Since glycosides lack a glycosidic hydroxyl, they are not capable of tautomerism, i.e. formation of a linear form containing an aldehyde group. Glycosides do not react with ammonia solution of silver oxide and Fehling liquid. However, in an acidic environment, glycosides are easily hydrolyzed to form the parent compounds:
Under the action of enzyme systems of microorganisms, monosaccharides can be transformed into various other organic compounds. Such reactions are called fermentation. The alcoholic fermentation of glucose is widely known, resulting in the formation of ethyl alcohol. Other types of fermentation are also known, for example, lactic acid, butyric acid, citric acid, glycerin.
Carbohydrates are part of the cells and tissues of all plant and animal organisms. They are of great importance as sources of energy in metabolic processes.
Carbohydrates serve as the main ingredient in mammalian food. Their well-known representative - glucose - is found in plant juices, fruits, fruits and especially in grapes (hence its name - grape sugar). It is an essential component of the blood and tissues of animals and a direct source of energy for cellular reactions.
Carbohydrates are formed in plants during photosynthesis from carbon dioxide and water. For humans, the main source of carbohydrates is plant foods.
Carbohydrates are divided into monosaccharides And polysaccharides. Monosaccharides do not hydrolyze to form simpler carbohydrates. Polysaccharides capable of hydrolysis can be considered as polycondensation products of monosaccharides. Polysaccharides are high-molecular compounds whose macromolecules contain hundreds and thousands of monosaccharide residues. The intermediate group between mono- and polysaccharides consists of oligosaccharides(from Greek oligos- a little), having a relatively small molecular weight.
A component of the above names - saccharides- is associated with the common name of carbohydrates that is still used - Sahara.
11.1. Monosaccharides
11.1.1. Structure and stereoisomerism
Monosaccharides are generally solids that are highly soluble in water, poorly soluble in alcohol, and insoluble in most organic solvents. Almost all monosaccharides have a sweet taste.
Monosaccharides can exist in both open (oxo form) and cyclic forms. In solution, these isomeric forms are in dynamic equilibrium.
Open forms.Monosaccharides (monoses) are heterofunctional compounds. Their molecules simultaneously contain carbonyl (aldehyde or ketone) and several hydroxyl groups, i.e. monosaccharides are polyhydroxycarbonyl compounds - polyhydroxyaldehydes And polyhydroxyketones. They have an unbranched carbon chain.
Monosaccharides are classified based on the nature of the carbonyl group and the length of the carbon chain. Monosaccharides containing an aldehyde group are called aldoses, and the ketone group (usually in position 2) - ketoses(suffix -ose used for the names of monosaccharides: glucose, galactose, fructose, etc.). In general, the structure of aldoses and ketoses can be represented as follows.
Depending on the length of the carbon chain (3-10 atoms), monosaccharides are divided into trioses, tetroses, pentoses, hexoses, heptoses, etc. The most common are pentoses and hexoses.
Stereoisomerism.Monosaccharide molecules contain several centers of chirality, which is the reason for the existence of many stereoisomers corresponding to the same structural formula. For example, aldohexose has four asymmetric carbon atoms and corresponds to 16 stereoisomers (2 4), i.e. 8 pairs of enantiomers. Compared to the corresponding aldoses, ketohexoses contain one less chiral carbon atom, so the number of stereoisomers (2 3) is reduced to 8 (4 pairs of enantiomers).
Open (non-cyclic) forms of monosaccharides are depicted in the form of Fischer projection formulas (see 7.1.2). The carbon chain in them is written vertically. In aldoses, an aldehyde group is placed at the top; in ketoses, a primary alcohol group is placed adjacent to the carbonyl group. The chain numbering begins with these groups.

The D,L system is used to indicate stereochemistry. The assignment of a monosaccharide to the D- or L-series is carried out according to the configuration of the chiral center farthest from the oxo group, regardless of the configuration of other centers! For pentoses, such a “determining” center is the C-4 atom, and for hexoses it is C-5. The position of the OH group at the last chirality center on the right indicates that the monosaccharide belongs to the D-series, on the left - to the L-series, i.e., by analogy with the stereochemical standard - glyceraldehyde (see 7.1.2).
It is known that the R,S system is universal for designating the stereochemical structure of compounds with several chirality centers (see 7.1.2). However, the cumbersome nature of the resulting names for monosaccharides limits its practical application.
Most natural monosaccharides belong to the D-series. Among aldopentoses, D-ribose and D-xylose are often found, and among ketopentoses, D-ribulose and D-xylulose are often found.
The common names for ketosis are formed by introducing the suffix -street in the names of the corresponding aldoses: ribose corresponds to ribulose, xylose - xylulose(from this rule the name “fructose” drops out, which has no connection with the name of the corresponding aldose).

As can be seen from the above formulas, stereoisomeric d-aldohexoses, as well as d-aldopentoses and d-ketopentoses, are diastereomers. Among them there are those that differ in the configuration of only one chirality center. Diastereomers that differ in the configuration of only one asymmetric carbon atom are called epimers. Epimers are a special case of diastereomers. For example, d-glucose and d-galactose are different
from each other only by the configuration of the C-4 atom, i.e. they are epimers at C-4. Similarly, d-glucose and d-mannose are epimers at C-2, and d-ribose and d-xylose are epimers at C-3.
Each d-series aldose corresponds to an l-series enantiomer with the opposite configuration of all chirality centers.

Cyclic forms. The open forms of monosaccharides are convenient for considering the spatial relationships between stereoisomeric monosaccharides. In fact, monosaccharides are structurally cyclic hemiacetals. The formation of cyclic forms of monosaccharides can be represented as the result of the intramolecular interaction of carbonyl and hydroxyl groups (see 9.2.2) contained in the monosaccharide molecule.

The hemiacetal hydroxyl group in carbohydrate chemistry is calledglycosidic.Its properties differ significantly from other (alcohol) hydroxyl groups.
As a result of cyclization, thermodynamically more stable furanose (five-membered) and pyranose (six-membered) cycles are formed. The names of the cycles come from the names of related heterocyclic compounds - furan and pyran.

The formation of these cycles is associated with the ability of the carbon chains of monosaccharides to adopt a rather favorable claw-shaped conformation (see 7.2.1). As a result, the aldehyde (or ketone) and hydroxyl groups at C-4 (or at C-5), i.e., those functional groups as a result of the interaction of which intramolecular cyclization occurs, appear to be close in space. If the hydroxyl group at C-5 of aldohexoses reacts, a hemiacetal with a six-membered pyranose ring appears. A similar cycle in ketohexoses is obtained with the participation of the hydroxyl group at C-6 in the reaction.

In the names of cyclic forms, along with the name of the monosaccharide, the size of the cycle is indicated in words pyranose or furanose. If the hydroxyl group at C-4 participates in cyclization in aldohexoses, and at C-5 in ketohexoses, then hemiacetals with a five-membered furanose ring are obtained.

In the cyclic form, an additional center of chirality is created - a carbon atom that was previously part of the carbonyl group (in aldoses this is C-1). This atom is called anomeric, and the two corresponding stereoisomers are α- and β-anomers(Fig. 11.1). Anomers are a special case of epimers.
Different configurations of the anomeric carbon atom arise due to the fact that the aldehyde group, due to a rotation around the C-1-C-2 σ bond, is attacked by the nucleophilic oxygen atom from virtually different sides (see Fig. 11.1). As a result, hemiacetals with opposite configurations of the anomeric center are formed.
For an α-anomer, the configuration of the anomeric center is the same as the configuration of the “terminal” chiral center, which determines its belonging to d- or l -series, and for the β-anomer it is the opposite. In Fischer projection formulas for monosaccharides d -series in the α-anomer the glycosidic group OH is located on right, and in the β-anomer - left from the carbon chain.

Rice. 11.1.Formation of α- and β-anomers using an example d-glucose
Haworth's formulas. Cyclic forms of monosaccharides are depicted in the form of Haworth's perspective formulas, in which the cycles are shown as flat polygons lying perpendicular to the plane of the drawing. The oxygen atom is located in the pyranose ring in the far right corner, in the furanose ring it is located behind the plane of the ring. The symbols for carbon atoms in the rings do not indicate.

To move to the Haworth formulas, the cyclic Fischer formula is transformed so that the oxygen atom of the cycle is located on the same straight line with the carbon atoms included in the cycle. This is illustrated below for a-d-glucopyranose by two rearrangements at the C-5 atom, which does not change the configuration of this asymmetric center (see 7.1.2). If the transformed Fischer formula is placed horizontally, as required by the rules for writing Haworth formulas, then the substituents located to the right of the vertical line of the carbon chain will be under the plane of the cycle, and those to the left will be above this plane.
d-aldohexoses in the pyranose form (and d-aldopentoses in the furanose form) have the group CH 2 OH is always located above the plane of the cycle, which serves as a formal sign of the d-series. The glycosidic hydroxyl group in a-anomers of d-aldoses appears under the ring plane, and in β-anomers it appears above the plane.

For the purpose of simplification, Haworth's formulas often do not depict the symbols of hydrogen atoms and their bonds with the carbon atoms of the cycle. If we are talking about a mixture of anomers or a stereoisomer with an unknown configuration of the anomeric center, then the position of the glycosidic group OH is indicated by a wavy line.
d-GLUCOPYRANOSE

The transition occurs in ketoses according to similar rules, as shown below using the example of one of the anomers of the furanose form of d-fructose.

11.1.2. Cyclo-oxo tautomerism
In the solid state, monosaccharides are in a cyclic form. Depending on the solvent from which d-glucose was recrystallized, it is obtained either as a-d-glucopyranose (from alcohol or water) or as β-d-glucopyranose (from pyridine). They differ in the specific rotation angle [a] D20, namely +112? at a-anomer and +19? at the β-anomer. For a freshly prepared solution
For each anomer, when standing, a change in specific rotation is observed until a constant rotation angle of +52.5° is reached, the same for both solutions.
The change in time of the angle of rotation of the plane of polarization of light by carbohydrate solutions is calledmutarotation.
The chemical essence of mutarotation is the ability of monosaccharides to exist in the form of an equilibrium mixture of tautomers - open and cyclic forms. This type of tautomerism is called cyclo-oxo-tautomerism.
In solutions, the equilibrium between the four cyclic tautomers of monosaccharides is established through the open form - the oxo form. The interconversion of a- and β-anomers into each other through an intermediate oxo form is called anomerization.
Thus, in solution, d-glucose exists in the form of tautomers: oxo forms and a- and β-anomers of pyranose and furanose cyclic forms.

The mixture of tautomers is dominated by pyranose forms. The oxo form, as well as tautomers with furanose rings, are present in small quantities. What is important, however, is not the absolute content of one or another tautomer, but the possibility of their transition into each other, which leads to the replenishment of the amount of the “necessary” form as it is consumed.
tion in any process. For example, despite the insignificant content of oxo form, glucose enters into reactions characteristic of the aldehyde group.
Similar tautomeric transformations occur in solutions with all monosaccharides and most known oligosaccharides. Below is a diagram of tautomeric transformations of the most important representative of ketohexoses - d-fructose, found in fruits, honey, and also included in sucrose (see 11.2.2).

11.1.3. Conformations
Haworth's visual formulas, however, do not reflect the real geometry of monosaccharide molecules, since five- and six-membered rings are not planar. Thus, the six-membered pyranose ring, like cyclohexane, adopts the most favorable chair conformation (see 7.2.2). In common monosaccharides, the bulky primary alcohol group CH 2 OH and most hydroxyl groups are in more favorable equatorial positions.
Of the two anomers of d-glucopyranose, the β-anomer predominates in solution, in which all substituents, including the hemiacetal hydroxyl, are located equatorially.

The high thermodynamic stability of d-glucopyranose, due to its conformational structure, explains the greatest distribution of d-glucose in nature among monosaccharides.
The conformational structure of monosaccharides determines the spatial arrangement of polysaccharide chains, forming their secondary structure.
11.1.4. Non-classical monosaccharides
Non-classical monosaccharides are a number of compounds that have a common structural “architecture” with ordinary, “classical” monosaccharides (aldoses and ketoses), but differ either in the modification of one or more functional groups, or in the absence of some of them. Such compounds often lack the OH group. They are named by adding the prefix to the name of the original monosaccharide deoxy- (means the absence of an OH group) and the name of the “new” substituent.
Deoxysugars.The most common of the deoxy sugars, 2-deoxy-D-ribose, is a structural component of DNA. Natural cardiac glycosides (see 15.3.5) used in cardiology contain residues of dideoxy sugars, for example digitoxoses (digitalis cardiac glycosides).
Amino sugar.These derivatives, containing an amino group instead of a hydroxyl group (usually at C-2), have basic properties and form crystalline salts with acids. The most important representatives of amino sugars are analogues of d-glucose and d-galactose, for which semi-trivial sugars are often used.

The new names are d-glucosamine and d-galactosamine, respectively. The amino group in them can be acylated with acetic and sometimes sulfuric acid residues.
Aldites.Aldites, also called sugar alcohols, include polyhydric alcohols containing a hydroxyl group instead of an oxo group =O. Each aldose corresponds to one alditol, the name of which uses the suffix -it instead of -Ozya, for example d-mannitol (from d-mannose). Alditols have a more symmetrical structure than aldoses, so among them there are mesocompounds (internally symmetrical), such as xylitol.

Acid sugars.Monosaccharides in which instead of a CH unit 2 OH contains the group COOH, have a common name uronic acids. Their names use the combination -uronic acid instead of a suffix -Ozya the corresponding aldose. Note that the chain numbering is from the aldehyde carbon atom, and not from the carboxyl carbon atom, in order to preserve the structural relationship with the original monosaccharide.
Uronic acids are components of plant and bacterial polysaccharides (see 13.3.2).
ACID SUGAR

Monosaccharides containing a carboxyl group instead of an aldehyde group are classified as aldonic acids. If carboxyl groups are present at both ends of the carbon chain, then such compounds have the common name Aldaric acids. In the nomenclature of these types of acids, combinations are used, respectively -onic acid And - aronic acid.
Aldonic and aldaric acids cannot form tautomeric cyclic forms, since they lack an aldehyde group. Aldaric acids, like alditols, can exist in the form of meso-compounds (an example is galactaric acid).

Ascorbic acid (vitamin C). This, perhaps the oldest and most popular vitamin, is close in structure to monosaccharides and is a γ-lactone acid (I). Ascorbic acid
found in fruits, especially citrus fruits, berries (rose hips, black currants), vegetables, milk. Produced industrially on a large scale from d-glucose.

Ascorbic acid exhibits quite strong acidic properties (pK a 4.2) due to one of the hydroxyl groups of the enediol fragment. When salts are formed, the γ-lactone ring does not open.
Ascorbic acid has strong reducing properties. Formed during its oxidation dehydroascorbic acid easily reduced to ascorbic acid. This process provides a series of redox reactions in the body.

11.1.5. Chemical properties
Monosaccharides are substances with rich reactivity. Their molecules contain the following most important reaction centers:
Hemiacetal hydroxyl (highlighted);
Alcohol hydroxyl groups (all others except hemiacetal);
Carbonyl group of acyclic form.

Glycosides.Glycosides include derivatives of cyclic forms of carbohydrates in which the hemiacetal hydroxyl group is replaced by an OR group. The non-carbohydrate component of the glycoside is called aglycone. The connection between the anomeric center (in aldoses it is C-1, in ketoses it is C-2) and the OR group is called glycosidic. Glycosides are acetals of cyclic forms of aldoses or ketoses.

Depending on the size of the oxide cycle, glycosides are divided into pyranosides And furanosides. Glucose glycosides are called glucosides, ribose - ribosides, etc. In the full name of glycosides, the name of the radical R, the configuration of the anomeric center (α- or β-) and the name of the carbohydrate residue with the replacement of the suffix are successively indicated -ose on -ozide (See examples in the reaction scheme below).
Glycosides are formed by the interaction of monosaccharides with alcohols under acid catalysis; in this case, only the hemiacetal OH group enters into the reaction.

Solutions of glycosides do not mutarotate.
The transformation of a monosaccharide into a glycoside is a complex process that occurs through a series of sequential reactions. In general terms it is ana-
is logical for the preparation of acyclic acetals (see 5.3). However, due to the reversibility of the reaction, tautomeric forms of the original monosaccharide and four isomeric glycosides (α- and β-anomers of furanosides and pyranosides) can be in equilibrium in solution.
Like all acetals, glycosides are hydrolyzed by dilute acids, but are resistant to hydrolysis in a slightly alkaline environment. Hydrolysis of glycosides leads to the corresponding alcohols and monosaccharides and is the reverse reaction to their formation. Enzymatic hydrolysis of glycosides underlies the breakdown of polysaccharides in animal organisms.
Esters.Monosaccharides are easily acylated by organic acid anhydrides, forming esters with the participation of all hydroxyl groups. For example, when reacting with acetic anhydride, acetyl derivatives of monosaccharides are obtained. Esters of monosaccharides are hydrolyzed in both acidic and alkaline environments.

Esters of inorganic acids, in particular esters of phosphoric acid - phosphates, are of great importance. They are found in all plant and animal organisms and are metabolically active forms of monosaccharides. The most important role is played by d-glucose and d-fructose phosphates.

Esters of sulfuric acid - sulfates - are part of connective tissue polysaccharides (see 11.3.2).
Recovery.When monosaccharides (their aldehyde or ketone group) are reduced, alditols are formed.

Hexahydric alcohols -D-glucite(sorbitol) and D-mannitol- are obtained by reducing glucose and mannose, respectively. Alditols are easily soluble in water, have a sweet taste, and some of them (xylitol and sorbitol) are used as sugar substitutes for patients with diabetes.
When reducing aldoses, only one polyol is obtained, when reducing ketoses, a mixture of two polyols is obtained; for example, from d -fructose is formed d-glucite and d-mannitol.

Oxidation.Oxidation reactions are used to detect monosaccharides, in particular glucose, in biological fluids (urine, blood).
Any carbon atom in a monosaccharide molecule can undergo oxidation, but the aldehyde group of aldoses in an open form is most easily oxidized.
Mild oxidizing agents (bromine water) can oxidize the aldehyde group into a carboxyl group without affecting other groups. At
This produces aldonic acids. So, during oxidation d -glucose is obtained from bromine water d -gluconic acid. Its calcium salt, calcium gluconate, is used in medicine.

The action of stronger oxidizing agents, such as nitric acid, potassium permanganate, and even Cu 2 + or Ag + ions leads to a deep decomposition of monosaccharides with the rupture of carbon-carbon bonds. The carbon chain is preserved only in certain cases, for example during oxidation d-glucose in d -glucaric acid or d -galactose into galactaric (mucus) acid.

The resulting galactaric acid is sparingly soluble in water and precipitates, which is used to detect galactose by this method.
Aldoses are easily oxidized by copper(11) and silver complex compounds - Fehling's and Tollens' reagents, respectively (see also 5.5). Such reactions are possible due to the presence of the aldehyde (open) form in the tautomeric mixture.

Due to the ability to reduce Cu 2 + or Ag + ions, monosaccharides and their derivatives containing a potential aldehyde group are calledrestorative.
Glycosides do not exhibit reducing ability and do not give a positive test with these reagents. However, ketoses are capable of reducing metal cations, since in an alkaline environment they isomerize into aldoses.
Direct oxidation of the CH unit 2 OH of monosaccharides into a carboxyl group is impossible due to the presence of an aldehyde group that is more prone to oxidation; therefore, to convert a monosaccharide into uronic acid, a monosaccharide with a protected aldehyde group is subjected to oxidation, for example, in the form of a glycoside.

Formation of glucuronic acid glycosides - glucuronides- is an example of a biosynthetic process conjugation, i.e., the process of binding drugs or their metabolites with nutrients, as well as with toxic substances, followed by excretion from the body in the urine.

11.2. Oligosaccharides
Oligosaccharides are carbohydrates built from several monosaccharide residues (from 2 to 10) linked by a glycosidic bond.
The simplest oligosaccharides are disaccharides (bioses), which consist of residues of two monosaccharides and are glycosides (full acetals), in which one of the residues acts as an aglycone. The acetal nature is associated with the ability of disaccharides to hydrolyze in an acidic environment to form monosaccharides.
There are two types of binding of monosaccharide residues:
Due to the hemiacetal group OH of one monosaccharide and any alcohol group of another (in the example below - hydroxyl at C-4); this is a group of reducing disaccharides;
With the participation of hemiacetal OH groups of both monosaccharides; This is a group of non-reducing disaccharides.

11.2.1. Reducing disaccharides
In these disaccharides, one of the monosaccharide residues participates in the formation of a glycosidic bond due to the hydroxyl group (most often at C-4). The disaccharide contains a free hemiacetal hydroxyl group, as a result of which the ability to open the ring is retained.
The reducing properties of such disaccharides and the mutarotation of their solutions are due to cyclo-oxo-tautomerism.
Representatives of reducing disaccharides are maltose, cellobiose, and lactose.
Maltose.This disaccharide is also called malt sugar (from lat. maltum- malt). It is the main product of the breakdown of starch under the action of the β-amylase enzyme, secreted by the salivary gland, and also contained in malt (sprouted, then dried and crushed cereal grains). Maltose has a less sweet taste than sucrose.

Maltose is a disaccharide in which the residues of two d-glucopyranose molecules are linked by an a(1^4)-glycosidic bond.
The anomeric carbon atom involved in the formation of this bond has an a-configuration, and an anomeric atom with a hemiacetal hydroxyl group can have both an α- and β-configuration (a- and β-maltose, respectively).
In the systematic name of a disaccharide, the “first” molecule acquires the suffix -zil, and the “second” retains the suffix -osa. In addition, the full name indicates the configurations of both anomeric carbon atoms.
Cellobiose.This disaccharide is formed by incomplete hydrolysis of cellulose polysaccharide.
Cellobiose is a disaccharide in which the residues of two d-glucopyranose molecules are linked by a β(1-4)-glycosidic bond.
The difference between cellobiose and maltose is that the anomeric carbon atom involved in the formation of the glycosidic bond has a β configuration.

Maltose is broken down by the enzyme α-glucosidase, which is not active against cellobiose. Cellobiose can be broken down by the enzyme β-glucosidase, but this enzyme is absent in the human body, so cellobiose and the corresponding polysaccharide cellulose cannot be processed in the human body. Ruminants can feed on cellulose (fiber) from grasses because the bacteria in their gastrointestinal tracts have β-glucosidase.
The configurational difference between maltose and cellobiose also entails a conformational difference: the α-glycosidic bond in maltose is located axially, and the β-glycosidic bond in cellobiose is equatorial. The conformational state of disaccharides is the root cause of the linear structure of cellulose, which includes cellobiose, and the coil-like structure of amylose (starch), built from maltose units.

Lactosefound in milk (4-5%) and obtained from whey after separating the curd (hence its name “milk sugar”).
Lactose is a disaccharide in which d-galactopyranose and d-glucopyranose residues are linked by a P(1-4)-glycosidic bond.
The anomeric carbon atom of d-galactopyranose involved in the formation of this bond has a β configuration. The anomeric atom of the glucopyranose moiety can have both α- and β-configuration (α- and β-lactose, respectively).
11.2.2. Non-reducing disaccharides
The most important of the non-reducing disaccharides is sucrose. Its sources are sugar cane, sugar beets (up to 28% of dry matter), plant and fruit juices.
Sucrose is a disaccharide in which the α-d-glucopyranose and β-d-fructofuranose residues are linked by glycosidic bonds due to the hemiacetal hydroxyl groups of each monosaccharide.


Since the sucrose molecule lacks hemiacetal hydroxyl groups, it is incapable of cyclo-oxo-tautomerism. Sucrose solutions do not mutate.
11.2.3. Chemical properties
In chemical essence, oligosaccharides are glycosides, and reducing oligosaccharides also have the characteristics of monosaccharides, since they contain a potential aldehyde group (in open form) and a hemiacetal hydroxyl. This determines their chemical behavior. They undergo many reactions characteristic of monosaccharides: they form esters and are capable of oxidation and reduction under the influence of the same reagents.
The most characteristic reaction of disaccharides is acid hydrolysis, leading to the cleavage of the glycosidic bond with the formation of monosaccharides (in all tautomeric forms). In general terms, this reaction is similar to the hydrolysis of alkyl glycosides (see 11.1.5).

11.3. Polysaccharides
Polysaccharides make up the bulk of organic matter in the Earth's biosphere. They perform three important biological functions, acting as structural components of cells and tissues, energy reserves and protective substances.
Polysaccharides (glycans) are high molecular weight carbohydrates. By chemical nature they are polyglycosides (polyacetals).
According to the principle of structure, polysaccharides do not differ from reducing oligosaccharides (see 11.2). Each monosaccharide unit is connected by glycosidic bonds to the previous and subsequent units. In this case, a hemiacetal hydroxyl group is provided for connection with the subsequent unit, and an alcohol group with the previous one. The difference lies only in the number of monosaccharide residues: polysaccharides can contain hundreds and even thousands of them.
In polysaccharides of plant origin, (1-4)-glycosidic bonds are most often found, and in polysaccharides of animal and bacterial origin there are bonds of other types. At one end of the polymer chain there is a reducing monosaccharide residue. Since its proportion in the entire macromolecule is very small, polysaccharides exhibit practically no reducing properties.
The glycosidic nature of polysaccharides determines their hydrolysis in acidic and stability in alkaline media. Complete hydrolysis leads to the formation of monosaccharides or their derivatives, while incomplete hydrolysis leads to a number of intermediate oligosaccharides, including disaccharides.
Polysaccharides have a large molecular weight. They are characterized by a higher level of structural organization of macromolecules, typical of high-molecular substances. Along with the primary structure, i.e., a certain sequence of monomeric residues, an important role is played by the secondary structure, determined by the spatial arrangement of the macromolecular chain.
Polysaccharide chains can be branched or unbranched (linear).
Polysaccharides are divided into groups:
Homopolysaccharides, consisting of residues of one monosaccharide;
Heteropolysaccharides, consisting of residues of different monosaccharides.
Homopolysaccharides include many polysaccharides of plant (starch, cellulose, pectin), animal (glycogen, chitin) and bacterial (dextrans) origin.
Heteropolysaccharides, which include many animal and bacterial polysaccharides, have been less studied but play an important biological role. Heteropolysaccharides in the body are associated with proteins and form complex supramolecular complexes.
11.3.1. Homopolysaccharides
Starch.This polysaccharide consists of two types of polymers built from d-glucopyranose: amylose(10-20%) and amylopectin(80-90%). Starch is formed in plants during photosynthesis and is “stored” in tubers, roots, and seeds.
Starch is a white amorphous substance. It is insoluble in cold water, but swells in hot water and some of it gradually dissolves. When starch is rapidly heated due to the moisture it contains (10-20%), hydrolytic cleavage of the macromolecular chain into smaller fragments occurs and a mixture of polysaccharides called dextrins. Dextrins are more soluble in water than starch.
This process of breaking down starch, or dextrinization, carried out during baking. Flour starch converted into dextrins is easier to digest due to its greater solubility.
Amylose is a polysaccharide in which d-glucopyranose residues are linked by a(1-4)-glycosidic bonds, i.e. the disaccharide fragment of amylose is maltose.

The amylose chain is unbranched, includes up to a thousand glucose residues, molecular weight up to 160 thousand.
According to X-ray diffraction analysis, the amylose macromolecule is coiled (Fig. 11.2). There are six monosaccharide units for each turn of the helix. Molecules of appropriate size, for example iodine molecules, can enter the internal channel of the helix, forming complexes called switching connections. The complex of amylose with iodine is blue. This is used for analytical purposes to discover both starch and iodine (starch iodine test).

Rice. 11.2.Helical structure of amylose (view along the axis of the helix)
Amylopectin, unlike amylose, has a branched structure (Fig. 11.3). Its molecular weight reaches 1-6 million.

Rice. 11.3.Branched macromolecule of amylopectin (colored circles are places of branching of side chains)
Amylopectin is a branched polysaccharide, in the chains of which D-glucopyranose residues are linked by a(1^4)-glycosidic bonds, and at branching points by a(1^6)-bonds. Between the branch points there are 20-25 glucose residues.

Hydrolysis of starch in the gastrointestinal tract occurs under the action of enzymes that break down a(1-4)- and a(1-6)-glycosidic bonds. The end products of hydrolysis are glucose and maltose.
Glycogen.In animal organisms, this polysaccharide is a structural and functional analogue of plant starch. It is similar in structure to amylopectin, but has even greater chain branching. Typically, between branch points there are 10-12, sometimes even 6, glucose units. Conventionally, we can say that the branching of the glycogen macromolecule is twice that of amylopectin. Strong branching helps glycogen perform its energy function, since only with a plurality of terminal residues can rapid cleavage of the required number of glucose molecules be ensured.
The molecular weight of glycogen is unusually large and reaches 100 million. This size of macromolecules helps perform the function of a reserve carbohydrate. Thus, the glycogen macromolecule, due to its large size, does not pass through the membrane and remains inside the cell until the need for energy arises.
Hydrolysis of glycogen in an acidic environment occurs very easily with a quantitative yield of glucose. This is used in tissue analysis for glycogen content based on the amount of glucose formed.
Similar to glycogen in animal organisms, amylopectin, which has a less branched structure, plays the same role as a reserve polysaccharide in plants. This is due to the fact that metabolic processes occur much more slowly in plants and do not require a rapid influx of energy, as is sometimes necessary for an animal organism (stressful situations, physical or mental tension).
Cellulose.This polysaccharide, also called fiber, is the most common plant polysaccharide. Cellulose has great mechanical strength and serves as a support material for plants. Wood contains 50-70% cellulose; Cotton is almost pure cellulose. Cellulose is an important raw material for a number of industries (pulp and paper, textiles, etc.).
Cellulose is a linear polysaccharide in which d-glucopyranose residues are linked by P(1-4)-glycosidic bonds. The disaccharide moiety of cellulose is cellobiose.
The macromolecular chain has no branches; it contains 2.5-12 thousand glucose residues, which corresponds to a molecular weight from 400 thousand to 1-2 million.

The β-configuration of the anomeric carbon atom results in the cellulose macromolecule having a strictly linear structure. This is facilitated by the formation of hydrogen bonds within the chain, as well as between neighboring chains.

This packing of chains provides high mechanical strength, fibrousness, insolubility in water and chemical inertness, which makes cellulose an excellent material for building plant cell walls. Cellulose is not broken down by ordinary enzymes of the gastrointestinal tract, but is necessary for normal nutrition as a ballast substance.
The ether derivatives of cellulose are of great practical importance: acetates (artificial silk), nitrates (explosives, colloxylin) and others (viscose fiber, cellophane).
11.3.2. Heteropolysaccharides
Connective tissue polysaccharides. Among connective tissue polysaccharides, the most fully studied are chondroitin sulfates (skin, cartilage, tendons), hyaluronic acid (vitreous body of the eye, umbilical cord, cartilage, joint fluid), and heparin (liver). Structurally, these polysaccharides have some common features: their unbranched chains consist of disaccharide residues, which include uronic acid (d-glucuronic, d-galacturonic, l-iduronic - epimer of d-glucuronic acid at C-5) and amino sugar (N-acetylglucosamine, N-acetylgalactosamine). Some of them contain residues of sulfuric acid.
Connective tissue polysaccharides are sometimes called acidic mucopolysaccharides (from the Latin. mucus- mucus), since they contain carboxyl groups and sulfo groups.
Chondroitin sulfates. They consist of disaccharide residues of N-acetylated chondrosin linked by β(1-4)-glycosidic bonds.
N-Acetylchondrosin is built from residues D -glucuronic acid and N-acetyl-D -galactosamine linked by a β(1-3)-glycosidic bond.

As the name suggests, these polysaccharides are sulfuric acid esters (sulfates). The sulfate group forms an ester bond with the hydroxyl group of N-acetyl-D-galactosamine, located in position 4 or 6. Accordingly, chondroitin-4-sulfate and chondroitin-6-sulfate are distinguished. The molecular weight of chondroitin sulfates is 10-60 thousand.

Hyaluronic acid. This polysaccharide is built from disaccharide residues connected by β(1-4)-glycosidic bonds.

The disaccharide fragment consists of residues D -glucuronic acid and N-acetyl-D-glucosamine linkedβ (1-3)-glycosidic bond.
Heparin. In heparin, the repeating disaccharide units include residues of d-glucosamine and one of the uronic acids - d-glucuronic or l-iduronic. Quantitatively, l-iduronic acid predominates. Inside the disaccharide fragment there is an α(1-4)-glycosidic bond, and between the disaccharide fragments there is an α(1-4) bond if the fragment ends with l-iduronic acid, and a β(1-4) bond if d -glucuronic acid.
The amino group of most glucosamine residues is sulfated, and some of them are acetylated. In addition, sulfate groups are found at a number of l-iduronic acid residues (at position 2), as well as glucosamine (at position 6). The d-glucuronic acid residues are not sulfated. On average, there are 2.5-3 sulfate groups per disaccharide fragment. The molecular weight of heparin is 16-20 thousand.

Heparin prevents blood clotting, i.e. it exhibits anticoagulant properties.
Many heteropolysaccharides, including those discussed above, are found not in free form, but in bound form with polypeptide chains. Such high-molecular compounds are classified as mixed biopolymers, for which the term is currently used glycoconjugates.