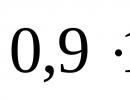Standard of mass in physics. Mass standard. Brief historical background. The storage conditions for the standard are kilograms. Communication equations. Using a mass standard to determine force, work, energy. Standard kilo is how much
The definition of the unit of mass - the kilogram - was given by the Third General Conference on Weights and Measures in 1901 as follows:
"The kilogram, a unit of mass, is represented by the mass of the international prototype of the kilogram."
When establishing the metric system of measures, the mass of 1 kg was adopted as a unit of mass, equal to the mass of 1 dm 3 of pure water at the temperature of its highest density (4 o C).
During this period, precise measurements of the mass of a known volume of water were made by successively weighing in air and water an empty bronze cylinder, the dimensions of which were carefully determined.
Based on these weighings, the first prototype of the kilogram was a platinum cylindrical weight with a height of 39 mm, equal to its diameter. It was deposited in the National Archives of France.
In the 19th century a repeated careful measurement of the mass of 1 dm 3 of water was made, and it was found that this mass was slightly (approximately 0.28 g) less than the mass of the Archive prototype.
In order to avoid changing the value of the unit of mass during further, more accurate weighings, the International Commission on Standards of the Metric System in 1872 decided to take the mass of the prototype kilogram of the Archive as the unit of mass.
In 1883, 42 prototype kilograms were made from a platinum-iridium alloy (90% platinum and 10% iridium) by Johnson, Matthay and Co., and copies No. 12 and No. 26 were received by lot by Russia in 1889 according to the Metric Convention. The standard is stored on a quartz stand under two glass caps in a steel cabinet in a special safe located in a thermostated room at the State Enterprise VNIIM im. D.I.Mendeleev”, St. Petersburg.
The state primary standard of a unit of mass, in addition to the weight, includes standard scales number 1 (Ruprecht) and number 2 (VNIIM) for 1 kg with remote control, which serve to transfer the size of the mass unit from prototype number 12 to copy standards and from copy standards to working standards ( 2 standards once every 10 years).
The error in reproducing mass using a kilogram standard does not exceed 2·10 -9. Thus, the kilogram standard allows you to record the result of a mass measurement in, at best, a number of nine digits. Despite all the precautions, as the results of international comparisons show, over 90 years the mass of the standard weight has increased by 0.02 mg. This is explained by the adsorption of molecules from the environment, the settling of dust on the surface of the weight and the formation of a thin corrosion film.
In connection with the development of work on the creation of new standards of PV units based on atomic constants, it is proposed to use the neutron mass as a standard. Another proposal is based on reproducing a unit of mass through a countable number of atoms of some chemical element, for example the isotope silicon-28. To do this, it is necessary to increase the accuracy of determining the Avogadro number, which is currently the focus of efforts of many laboratories around the world.
Related information:
- Lt;question>Measurement device designed to reproduce or store a unit of value
- V1: Characteristics of measured quantities, units of measurement and measurement errors
- A. In kilograms. B. In grams. B. In atomic mass units (a.m.u.). G. V MeV
- A. The same. B. The mass of the shot is slightly greater than the mass of the weight. B. The mass of the shot is slightly less than the mass of the weight. D. This experience does not provide a basis for answering the question asked.
Probably, many readers remember the television advertisement of one mobile operator, in which the famous slogan “How much is it in grams?” appeared. “Precision is never superfluous,” one of the heroes summed up his question roller. In fact, he was cunning - it is impossible to accurately weigh, say, 200 grams of something. And it’s not just that existing weighing methods are bad - it’s just that people don’t have a reliable standard for a kilogram, and therefore a gram.
The need to develop standards, based on which it is possible to determine the values of mass, time, length and temperature (and after the advent of physics, the intensity of light, the intensity of current and a unit of matter) arose among humanity a long time ago. This need is quite understandable - in order to build roads and houses, travel and trade, constant units were needed, using which two builders or traders could understand what was drawn in each other’s drawings and what quantities of goods were being discussed.
Each civilization had its own units of measurement: for example, in Ancient Egypt, mass was measured in kantars and kikkars, in Ancient Greece - in talents and drachmas, and in Rus' - in poods and zolotniks. As scientists like to say, when creating each of these units, people seem to agreed, that from now on the mass, length or temperature of something will be compared to one unit of mass, length or temperature, respectively. The number of those who directly participated in these agreements was very small - the poods of two traders from different parts of the country could easily differ by a third.
How would an agreement worked great until people began to seriously engage in science and master engineering. It turned out that approximate values are not enough to describe the laws of nature or create a steam boiler, especially if people from different countries take part in the work. Realizing this fact, scientists from all over the world began to develop uniform, accurate standards, or standards, for basic units of measurement. On May 20, 1875, an agreement was signed in France to establish these units - the Metric Convention. All countries that signed this document committed to using specially created standards as standards. To provide signatory states with the most accurate standards, the International Chamber of Weights and Measures (or International Bureau of Weights and Measures) was created. The tasks of this organization include regular comparison of national standards with each other and supervision of work to create more accurate measurement methods.
In Russia, the introduction of the metric system is associated with the name of Dmitry Ivanovich Mendeleev, who created the Main Chamber of Weights and Measures in 1893 and generally did a lot for the development of metrology. He explained his interest in precise measurements as follows: “Science begins as soon as they begin to measure. Exact science is unthinkable without measure.” Thanks to the efforts of Mendeleev, from January 1, 1900, in Russia, along with national ones, metric measures were allowed to be used.
After the signing of the Metric Convention, experts began developing common standards for the meter and kilogram (these units of measurement existed before 1875, but there were no standards that were recognized throughout the world). The standard meter was established after the famous expedition to measure the length of the arc of the Paris meridian and was a ruler made of an alloy of platinum and iridium in a ratio of 9 to 1, the length of which was equal to one forty millionth of the meridian. Based on the location where it was stored, it began to be called “archive meter” or “archive meter.” The kilogram standard was cast from the same alloy, and its mass corresponded to the mass of one cubic decimeter (liter) of pure water at a temperature of 4 degrees Celsius (when water is at its maximum density) and standard atmospheric pressure at sea level. In 1889, during the first General Conference on Weights and Measures, a system of measures was adopted based on the newly produced standards of the meter and kilogram, as well as the standard of the second. The standard for a second began to be considered 1/86400 of the duration of an average solar day (later the standard was tied to the tropical year - a second was equated to 1/31556925.9747 of its part). Countries that recognized the new system of measures received copies of these standards, and the prototypes were sent to the Chamber of Weights and Measures for storage.
After some time, the standards of candela (light intensity), ampere (current intensity) and kelvin (temperature) were added to these three standards. In 1960, the Eleventh General Conference on Weights and Measures adopted a system of weights and measures based on the use of these six units and the mole (a unit of quantity of a substance - there is no standard for it) - the new system was called the International System of Units, or SI. It would seem that this was where the history of standards should have ended, but in reality, it was just beginning.
Everything that can go wrong...
As measurement technology improved, it became clear that all the standards stored in Paris were not ideal. Gradually, scientists came to the conclusion that it was worth taking not man-made objects as the standards for basic units, but much more advanced examples already created by nature. Thus, the standard second was taken to be a time interval equal to 9192631770 periods of radiation corresponding to the transition between two hyperfine levels of the ground (quantum) state of the cesium-133 atom at rest at 0 kelvin in the absence of disturbance by external fields, and the standard meter was the distance that light travels in a vacuum in a period of time equal to 1/299792458 of a second. Unlike the old ones, the new standards are atomic or quantum, that is, the most “basic” laws of nature “work” in them.
Gradually, six of the seven basic SI units received reproduction methods that did not require a unique standard stored somewhere in one place. Theoretically, any scientist who wants to know exactly (very accurately), for example, how long a second lasts, can take a milligram or two of the cesium-133 isotope and count when 919,263,1770 periods of radiation occur (by the way, their own atomic time standards are established, for example, at all GPS satellites). Only a kilogram of “in girls” remains - its standard is still collecting dust in a deep basement near Paris.
The word “gathering dust” in the previous paragraph is not a stylistic decoration at all - dust is in fact gradually accumulating on the kilogram standard, despite all countermeasures. It is impossible to take out a platinum-iridium cylinder and wipe it - firstly, when removing it, dust will again settle on it, and secondly, wiping or even fanning with a brush will inevitably lead to several molecules “bouncing off”. In other words, no matter what is done or not done to the standard, its mass changes over time. For a long time it was believed that these changes were insignificant, but a check carried out several years ago showed that recently the standard had “lost weight” by 50 micrograms, and this is already an impressive loss.
Mole, silicon and gold
A possible way out of this sad situation (over the next billion years the standard will become one third lighter) was proposed in 2007 by two American scientists from the Georgia Institute of Technology. Instead of a changeable cylinder, they proposed to consider a cube of carbon, which would contain a strictly defined number of atoms, as the standard of mass. Since the mass of each individual atom is constant, the mass of their aggregate will also not change. The researchers calculated that a cube weighing exactly one kilogram would consist of 2250 x 28148963 3 atoms (50184513538686668007780750 atoms), and its edge would be 8.11 centimeters. Over the course of three years, scientists clarified some details and presented their thoughts in an article, a preprint of which can be found on the website arXiv.org.
American physicists were concerned with the problem of the kilogram standard and chose carbon as the “reference” element for a reason - before that they were working on refining Avogadro’s number, one of the fundamental constants that determines how many atoms are contained in one mole of any substance. Although this number is one of the most important in chemistry, its exact meaning does not exist (among other questions, scientists, for example, decided whether it was even or not). Avogadro's number is chosen so that the mass of a mole in grams is equal to the mass of a molecule (atom) in atomic mass units. A carbon atom has a mass of 12 atomic mass units, which means the mass of a mole of carbon must be 12 grams. By refining Avogadro's number and taking it equal to 84446886 3 (602214098282748740154456), the researchers were able to calculate the required number of carbon atoms in the standard.
It is possible that the new work will be considered at the next General Conference on Weights and Measures, which will be held in 2011. However, scientists from Georgia have competitors. For example, the National Institute of Standards and Technology in Washington is very actively working on the concept of the electronic kilogram. Briefly, the essence of the method they propose is as follows: the standard is determined through the current strength, which is necessary to create a magnetic field capable of balancing a load of one kilogram. This method is very good because it allows you to achieve high accuracy (it is based on the use of another fundamental constant - Planck's constant), but the experiment itself is extremely complex.
Another version of the new standard is a silicon sphere, the parameters of which are calculated in such a way that it will contain a strictly defined number of atoms (this calculation can be carried out, since scientists know the distance between individual atoms, and the process of producing pure silicon is very well established). Such a sphere was even created, but difficulties immediately arose with it, reminiscent of the difficulties of the current standard - over time, the sphere loses some of its atoms and, in addition, a film of silicon oxide forms on it.
The third approach to creating a standard assumes that it will be produced every time de novo. To obtain a mass standard, it is necessary to accumulate bismuth and gold ions until their total charge reaches a certain value. This method has already been recognized as unsatisfactory: it takes too much time and the results are poorly reproducible. In general, with a high probability, all the described methods for obtaining a new kilogram standard, except for the method based on the use of Avogadro’s number, will remain only in the memory of historians of science, since, unlike the others, the kilogram standard in the form of a cube from the carbon-12 isotope is based on direct using one of the fundamental atomic concepts.
It is unclear whether the carbon standard will become generally accepted or whether scientists will come up with a new, more convenient way. But there is no doubt that the cylinder stored in Paris, which faithfully served people for 120 years, will soon retire.
In 1872, by decision of the International Commission on Standards of the Metric System, the mass of the prototype kilogram, stored in the National Archives of France, was adopted as a unit of mass. This prototype is a platinum cylindrical weight with a height and diameter of 39 mm. Prototypes of the kilogram for practical use were made from a platinum-iridium alloy. A platinum-iridium weight, closest to the mass of the Archive’s platinum kilogram, was adopted as the international prototype of the kilogram. It should be noted that the mass of the international prototype kilogram is somewhat different from the mass of a cubic decimeter of water. As a result, the volume of 1 liter of water and 1 cubic decimeter are not equal to each other (1 liter = 1.000028 dm 3). In 1964, the XII General Conference on Weights and Measures decided to equate 1 l to 1 dm 3.
The international prototype of the kilogram was approved at the First General Conference on Meters and Weights in 1889 as a prototype of a unit of mass, although at that time there was no clear distinction between the concepts of mass and weight and therefore the mass standard was often called the weight standard.
By decision of the First Conference on Weights and Measures, platinum-iridium kilogram prototypes No. 12 and No. 26 were transferred to Russia from 42 kilogram prototypes produced. The kilogram prototype No. 12 was approved in 1899 as an optional state standard of mass (the pound had to be periodically compared with the kilogram) , and prototype No. 26 be used as a secondary standard.
The standard includes:
a copy of the international prototype of the kilogram (No. 12), which is a platinum-iridium weight in the form of a straight cylinder with rounded ribs with a diameter and height of 39 mm. The prototype of the kilogram is stored at VNIIM. D. M. Mendeleev (St. Petersburg) on a quartz stand under two glass covers in a steel safe. The standard is stored while maintaining the air temperature within (20 ± 3) ° C and relative humidity 65%. In order to preserve the standard, two secondary standards are compared with it every 10 years. They are used to further convey the size of a kilogram. When compared with the international standard kilogram, the domestic platinum-iridium weight was assigned a value of 1.0000000877 kg;
equal-arm prism scales 1 kg. No. 1 with remote control (in order to eliminate the operator’s influence on the ambient temperature), manufactured by Ruprecht, and equal-arm modern prism scales for 1 kg No. 2, manufactured at VNIIM. D.M. Mendeleev. Scales No. 1 and No. 2 serve to transfer the size of a unit of mass from prototype No. 12 to secondary standards.
Error in reproducing a kilogram, expressed by the standard deviation of the measurement result 2. 10 -9. The amazing durability of the standard unit of mass in the form of a platinum-iridium weight is not due to the fact that at one time the least vulnerable way to reproduce the kilogram was found. Not at all. Already several decades ago, the requirements for the accuracy of mass measurements exceeded the possibilities of their implementation using existing mass unit standards. Research into mass reproduction using the known fundamental physical mass constants of various atomic particles (proton, electron, neutron, etc.) has been ongoing for a long time. However, the real error in reproducing large masses (for example, a kilogram), tied, in particular, to the rest mass of the neutron, is so far significantly greater than the error in reproducing a kilogram using a platinum-iridium weight. The rest mass of a single particle - a neuron - is 1.6949286 (10)x10 -27 kg and is determined with a standard deviation of 0.59. 10 -6.
More than 100 years have passed since the prototypes of the kilogram were created. Over the past period, national standards were periodically compared with the international standard. In Japan, special scales have been created using a laser beam to record the “swing” of a rocker arm with a reference and tare weights. The results are processed using a computer. At the same time, the error in reproducing a kilogram was increased to approximately 10 -10 (according to standard deviation). One set of similar scales is available in the Metrological Service of the Armed Forces of the Russian Federation.
The kilogram is defined as the mass of the international standard kilogram kept by the International Bureau of Weights and Measures, which is a cylinder with a diameter and height of 39 mm made of a platinum-iridium alloy (90% platinum, 10% iridium). Initially, in 1793, the chemist Antoine Lavoisier and crystallographer Rene Juste Ailly proposed to the French Commission of Weights and Measures to use a gram as a unit of mass - the mass of one cubic centimeter of pure water at the melting point of ice. For ease of practical use, the already mentioned Lenoir produced a standard copper weight weighing 1000 grams. Since 1795, the new unit of mass was called the kilogram. Four years later, physicist Louis Lefebvre-Guignot's proposal to weigh water at the temperature of its maximum density (4°C) was accepted. The new kilogram standard was made of platinum and deposited in the Archives of the Republic. Several copies of it were also made for use as samples in the making of weights. However, measurements made in the 19th century showed that the mass of 1 dm 3 of water is 0.028 g less than the mass of the archival standard. In order to prevent any discrepancies in the future, the International Commission for Standards of the Metric System in 1872 decided to adopt the mass of the prototype, the Archive kilogram, as a unit of mass.
In 1880, the international standard of the kilogram from an alloy consisting of platinum and iridium was released, and four of the six currently existing official copies of this standard were made at the same time.
All of them are now stored under two sealed glass covers in a safe located in the basement of the International Bureau of Weights and Measures (BIPM) in Sèvres near Paris. In 1889, the 1st General Conference on Weights and Measures adopted the definition of the kilogram as equal to the international standard mass. This definition is valid in our time. For information - the International Bureau of Weights and Measures, BIPM (French Bureau International des Poids et Mesures, BIMP) is a permanent international organization with headquarters located in the city of Sèvres (a suburb of Paris, France) . Established in 1875, along with the signing of the Meter Convention. The main task of the Bureau is to ensure the existence of a unified measurement system in all countries participating in this convention. The BIPM stores international standards of basic units and carries out international metrological work related to the development and storage of international standards and the comparison of national standards with international ones and among themselves.
A copy of the international standard is also stored in the Russian Federation, at the All-Russian Research Institute of Metrology named after. Mendeleev. Approximately once every 10 years, national standards are compared with international ones. These comparisons indicate that the national standards are accurate to approximately 2 µg. Since they are stored under the same conditions, there is no reason to believe that the international standard is more accurate. For various reasons, over a hundred years the international standard loses 0.00000003rd part of its mass. However, by definition, the mass of the international standard is exactly equal to one kilogram. Therefore, any changes in the actual mass of the standard lead to a change in the value of the kilogram. 
The kilogram is one of the seven basic quantities of the international system of SI units. The rest - meter, second, ampere, kelvin, mole and candela - are not tied to specific material media. The platinum-iridium meter standard was canceled in 1960. The only currently remaining "mechanical" standard is the kilogram. But even the mass of the main international standard changes over time - by now it is believed that it has “lost weight” by 50 micrograms due to microtransfer of the substance to the surface of the stand during storage, as well as to the surface of the grips with which it is moved during comparison with national standards.
All this can distort the results of ultra-precise scientific calculations, so scientists are thinking about the need to redefine the kilogram. In 1975, Dr. Brian Kibble of the UK's National Physical Laboratory (NPL) proposed the idea of so-called watt balances. This device allows units of electrical and mechanical power to be interconnected. “This connection is the basis of metrology,” explains the leading researcher at the All-Russian Research Institute of Metrology. D. I. Mendeleev Edmund French. - The balance consists of two coils that interact with each other when an electric current flows. Unlike current balances, additional calibration is used here when the coil moves at a known speed in a reference magnetic field. Due to this, it is possible to significantly reduce the error in measuring the interaction force due to the geometry of the coil. Thus, it is possible to express the kilogram in terms of electrical units measured on the basis of quantum effects, that is, through fundamental constants - this will allow us to get rid of the “mechanical” standard. So far, working watt scales have been implemented in the USA at NIST and at NPL, but at the moment the smallest error in their measurements is 3.6 × 10 –8, which is at least two times worse than what is needed for the standard.”
Another way to redefine the kilogram was proposed by a group of scientists from Germany, Australia, Italy and Japan, led by researchers from the Physicotechnical Institute of Germany. They intend to use the “Avogadro method,” that is, define a kilogram as the nth number of atoms. “The main difficulties of this method are that you need to build an ideal crystal lattice,” says Edmund French, “without a single defect, and, moreover, from one isotope - silicon-28. The relative error of this method is still too high - 3.1×10 –7. By the way, there was another direction that was being developed here at VNIIM and in Japan - the method of levitating superconducting mass, which provided an accuracy of the order of 4 × 10 –6. But for various reasons, the studies were not completed in any of the countries.”
So the kilogram remains the last purely mechanical standard for now.
For your information, the permissible absolute error of a widely used 1 kilogram weight is 0.5 grams.
Based on materials from the sites: www.omedb.ru; www.russianamerica.com; wikipedia.org.
Kilogram(symbol: kg, kg) - a unit of mass, one of the basic SI units [system of units/measurements].
Currently, the kilogram is the only SI unit that is defined using an object made by people. All other units are now defined using fundamental physical properties and laws.
The standard was made in 1889 and has since been stored in International Bureau of Weights and Measures*
(located in Sevres near Paris) and is a cylinder with a diameter and height of 39.17 mm made of a platinum-iridium alloy (90% platinum, 10% iridium). It is stored under three sealed glass caps. The kilogram was originally defined as the mass of one cubic decimeter (liter) of pure water at a temperature of 4°C and standard atmospheric pressure at sea level.
Exact official copies of the international standard were also produced, which are used as national kilogram standards. In total, more than 80 copies were created. Copies of the international standard are also stored in the Russian Federation, at the All-Russian Research Institute of Metrology. Mendeleev. Approximately once every 10 years, national standards are compared with international ones. These comparisons indicate that the national standards are accurate to approximately 2 µg. Since they are stored under the same conditions, there is no reason to believe that the international standard is more accurate. For various reasons, over a hundred years, the international standard loses 3x10 −8 of its mass. However, by definition, the mass of the international standard is exactly equal to one kilogram. Therefore, any changes in the actual mass of the standard lead to a change in the value of the kilogram.
To eliminate these inaccuracies, various options are currently being considered to redefine the kilogram based on fundamental physical laws.
Also, since 2003, an international group of researchers from 8 countries, including Germany, Australia, Italy and Japan, under the auspices of the German standards laboratory, has been working to redefine the kilogram as the mass of a certain number of atoms of the silicon-28 isotope. The second project, called “Electronic Kilogram”, began in 2005 at (NIST). The leader of this project, Richard Steiner, claims that he has been working on the creation of an “electronic kilogram” for more than ten years. Scientists led by Dr. Steiner have created a device that measures the power required to generate an electromagnetic field that can lift one kilogram of mass. With its help, scientists were able to determine the mass of one kilogram with an accuracy of 99.999995%, they write on Wikipedia.
Scientists are moving closer to a non-physical description of the kilogram after discovering that the metal standard used as an international standard has begun to inexplicably lose weight.
The researchers say they still have some way to go before a definition is given, but if successful it would lead to the adoption of a new international standard used to define the kilogram.
Scientists say that it is the description of the kilogram that is so important, since it is the basic physical unit of scales, from which all others are already calculated as derivatives. Now the equivalent of a kilogram is a metal bar weighing about 2.2 British pounds [...] .
However, in 2007, it was found that the standard began to lose weight, in particular, scientists determined that a kilogram block began to weigh 50 micrograms less, several dozen exact copies. That is, we can say that the standard has lost weight comparable to the weight of a grain of sand. In this regard, physicists suggest that the block may continue to lose its weight.
Moreover, scientists say that other fundamental units such as ampere, volt, mole, meter and others are not tied to any physical references.
Earlier, German specialists from the National Institute of Metrology in Braunschweig reported that they would use a new 10-centimeter silicon sphere as a kilogram standard. According to scientists, the new standard is more accurate and stable than the one currently used.
The goal of the new project is to create a more reliable standard whose accuracy is measured at the atomic level. Scientists say that silicon atoms are ideal for this project because they are very stable and their compounds are almost indestructible under standard conditions.
It is noteworthy that the new silicon kilogram standard was partially developed in Russia. Scientists from Australia and Japan also took part in the project. In total, 2 million euros were spent on the production of a silicon sphere of unprecedented precision, and the process of its creation took just under 5 years.
According to Peter Becker, the project leader, to create a kilogram standard, physicists calculated how many silicon atoms should be in 1 kilogram of this element, after which they began to “assemble” the standard. However, Becker emphasizes that the new sphere is not ideally accurate, since today’s science is not able to fold a macro object in the literal sense of the word, collecting it atom by atom, writes ZN.UA based on materials from CyberSecurity.
* Help: What is the International Bureau of Weights and Measures?
Established in 1875, along with the signing of the Meter Convention. The main task of the Bureau is to ensure the existence of a unified measurement system in all countries participating in this convention.
The BIPM stores international standards of basic units and carries out international metrological work related to the development and storage of international standards and the comparison of national standards with international ones and among themselves.
The BIPM also conducts research in the field of metrology aimed at increasing the accuracy of measurements.
Over the years, the bureau was headed by famous European scientists: G. Govi (Italy, 1875-1877), J. Pernet (Switzerland, 1877-1879), O.-J. Broch (Norway, 1879-1889), J.-R. Benoit (France, 1889-1915), C.-E. Guillaume (Switzerland, 1915-1936), A. Perard (France, 1936-1951), C. Volet (Switzerland, 1951-1961) J. Terrien (France, 1962-1977), P. Giacomo (France, 1978-1988), T. J. Quinn (UK, 1988-2003).
From 2004 to the present, the director of the BIPM is Professor Andrew Wallard ( A. J. Wallard), Great Britain. The Bureau is financed by member countries of the Meter Convention.
There is also Main Chamber of Weights and Measures, which was established in 1893 in St. Petersburg on the initiative of D.I. Mendeleev, the scientist-custodian of the Depot of Model Weights and Measures, which was transformed into the Main Chamber.
The Main Chamber of Weights and Measures was the central institution of the Ministry of Finance, in charge of the verification department in the Russian Empire and subordinate to the trade department.
According to the Regulations on Weights and Measures of 1899, the task of the Chamber was “to maintain uniformity, fidelity and mutual correspondence of weights and measures”; according to the law of 1901, she was entrusted with the management of local calibration tents, their temporary departments, the distribution of verifiers who were attached to the Chamber, their secondment, etc., as well as resolving various issues in metrology and maintaining reports on the receipt of fees for marking measures to the treasury and scales. In the Chamber itself, the verification system was brought to the highest possible scientific and technical perfection.
Today, VNIIM is one of the world's largest centers of scientific and practical metrology, the country's leading organization for fundamental research in metrology and the main center of state standards in Russia. Subordinate to the Federal Agency for Technical Regulation and Metrology.
In July 1994, by Decree of the Government of the Russian Federation, VNIIM was awarded the status of the State Scientific Center of the Russian Federation. As a State Scientific Center of the Russian Federation, VNIIM is subordinate to the Ministry of Education and Science of Russia and is part of the Association of State Scientific Centers, they write on Wikipedia.