Minerals: Coal. Interesting facts about coal. Interesting facts about coal. Coal deposit Coal facts
Coal is the remains of plants that died many millions of years ago, the decay of which was interrupted as a result of the loss of air supply. Therefore, they could not release the carbon taken from it into the atmosphere. The access of air ceased especially abruptly where swamps and swampy forests sank as a result of tectonic movements and changes in climatic conditions and were covered with other substances. At the same time, plant remains were transformed under the influence of bacteria and fungi (carbonized) into peat and further into brown, stone, anthracite and graphite.
Coal became the first fossil fuel used by humans. The use of coal in the modern world is diverse. It is used to obtain electrical energy (energy), as a raw material for the metallurgical (coking) and chemical industries, for the production of rare and trace elements, and for the production of graphite.
Mining in Russia began in the second half of the 15th century under the Grand Duke of Moscow Ivan III. In 1491, the first Russian expedition went to the Pechora region to look for minerals. This expedition discovered deposits of silver and copper ores on the Tsilma River, a copper mine was built, and Russia began minting coins from its own metal.
The total geological (forecast) coal reserves in Russia are 4 trillion tons, which is 30% of the world's coal reserves. Explored (balance) reserves are estimated at 190 billion tons. The volume of production is limited by the total production capacity of mining enterprises.
Combustion (hydrogenation) of coal to form liquid fuel is promising. To produce 1 ton of oil, 2-3 tons of coal are consumed. Artificial graphite is obtained from coal. They are used as inorganic raw materials. When processing hard coal, vanadium, germanium, sulfur, gallium, molybdenum, zinc, and lead are extracted from it on an industrial scale. Ash from coal combustion, mining and processing wastes are used in the production of building materials, ceramics, refractory raw materials, alumina, and abrasives. In order to optimally use coal, it is enriched (removing mineral impurities).
Canaries are very sensitive to the methane content in the air. This feature was once used by miners who, going underground, took with them a cage with a canary. If singing had not been heard for a long time, then it was necessary to quickly go upstairs.
The age of the oldest coals is estimated at approximately 300-400 million years.
In the past, many aromatic components were extracted from coal.
The Japanese used it to make the famous samurai swords.
Mining began in one of the Dutch mines back in 1113. This mine is still in operation, and until recently it was where a very significant part of Dutch coal was mined.
Young and green. The allegorical expression does not suit brown coal. Geologists classify it as a young rock. Brown coal on Earth is approximately 50,000,000 years old. Accordingly, the breed was formed in the Tertiary period.
It includes the Paleogene and Neogene eras. In other words, brown coal was formed when the first people were already walking on the planet. However, despite its youth, the breed is not at all green. Its color is clear from the name. We will look into what causes the brown paint below.
Properties of brown coal
The color of brown coal is due to its base. This is plant matter, mainly wood. It is clearly visible in the lingites. A number of geologists consider them a separate rock, while others classify them as a variety brown coal. In Russia adhere to the latter point of view.
Be that as it may, it is decomposed vegetation. In , when it was lush and the trunks were gigantic, it settled at the bottom of the swamps. There, in conditions of oxygen deficiency, organic matter began to decompose. So in lingites the process is at the initial stage, you can still see pieces of wood. It is perishable, but the structure of the fibers can be traced.
Classic brown coal is a homogeneous mass. It is already difficult to distinguish wood fibers in it. However, the organic matter has not yet decomposed to the state of pure organic matter. Therefore, the brown color of the mass is preserved.
The presence of large particles in it causes the friability of the fossil. There is only 1 gram of mass per cubic centimeter of rock. It contains no more than 60 percent carbohydrates, and often only half.
Both density and saturation of the rock with hydrocarbons are responsible for energy intensity. Brown coal - fuel lower category. It is used, as a rule, in subsidiary farming. Industrialists need energy-intensive fuel that burns almost 100%. After burning the hero of the article, a lot of ash remains.
Use of brown coal– this is the settling of soot on the chimney, flame, acrid smoke. Ignition is facilitated by volatile substances, of which there are about 10% in brown coal. Another 30% comes from water, oxygen,... All this is unnecessary for fuel.

Characteristics of brown coal on the cut - “like a clod of earth.” However, what makes a rock like this is the presence of water. Once it evaporates, the fossil crumbles into dust. In other words, there are not enough viscous hydrocarbons to cement the rock particles.
Industrialists compress them. Without water use of brown coal little more effective. In its usual form, the combustion of 1 kilogram of rock produces no more than 10,000 kilocalories. The average is 5,500 kilocalories.
How is brown coal different from hard coal?
If the maximum age of brown coal is 50,000,000 years, then stone coal is 350,000,000 years old. In other words, the most ancient rock samples were formed back in the Devonian period. The vegetation then consisted mainly of giant horsetails, and they were also hidden in the seas.
There were 9 geological eras left until the 21st century. For them, the plant remains decomposed and were compressed so much that they turned into real stone. There is no trace of the friability of brown coal. The stone version of the rock is real.

Brown coal in the photo
The wood color in charcoal has been replaced with a deep black. This is a 1st grade hydrocarbon paint. There are almost 100% of them in the breed. True, this applies to the last stage of development of coal. In ordinary hydrocarbons from 72 to 90 percent.
The mass of impurities can be determined at a glance. Anthracite, for example, shines on a fault. This radiance is called coal. Impurities dull the rock. Brown coal reserves, accordingly, are always matte. In contrast to their 10,000 kilocalories per kilogram of burned fuel, there are 61,000. This is the indicator of stone coal
Brown mining Coal mining is carried out from depths of up to a kilometer. Since Devonian times a large mass of earth has been layered. Accordingly, the stone version of the rock is extracted from depths of about 3 kilometers.
Due to the small amount of impurities, coal burns almost without residue, produces a minimum of soot, and does not burn in the usual sense. There are no pronounced flames. However, it takes more resources to heat up a dense stone than to set fire to a loose brown mass.
This is another reason why the breed is used only by industrialists. They have the ability to maintain the desired temperature. Burning brown coal is similar to working with wet firewood.
Brown coal deposits and mining
Brown coal deposits at a kilometer depth they are among the oldest in the world, those that are 50,000,000 years old. The main deposits are even younger, therefore, located higher.
In, for example, most brown coal seams are located 10-60 meters from the surface. This encourages open-pit mining. This method extracts 2/3 of domestic coal reserves.
By the way, they are distributed unevenly. 60% are in Siberia. The Soltomskoye field, for example, is being developed in Altai. Rock reserves amount to 250,000,000 tons. There is brown coal in the Kansk-Achinsk basin.

Brown coal mining
Rock deposits are called pools because of their “spill” underground. Coal is not veins among other rocks and not compact aggregates, but vast “pancakes”. They extend for tens and hundreds of kilometers. Thus, in the Kansk-Achinsk basin, only surface reserves are concentrated on an area of 45,000 square kilometers.
In Siberia there is also lignite pool"Lensky" It is being developed on the territory of Yakutia. The deposit also affects the Krasnoyarsk Territory. The total area of deposits is 750,000 square kilometers. They include more than 2,000,000,000,000 tons. Those who are confused by zeros are talking about trillions.
Buy brown coal from the Lenskoye field, despite its vastness, is more expensive than from the Kansko-Achinskoye or Soltomskoye field. The reason is the complexity of the rock occurrence in Yakutia.
The “pancake” of the fossil is torn and crushed in places, sometimes sinks underground, sometimes rises to the surface. Most of the last sections have already been developed. Mining from the depths is more expensive, which affects the final rock.
In the west of the country brown coal is mined in the Podmoskovny swimming pool. It also contains a stone variety. Actually, the deposit began to form in the Carboniferous period. It belongs to the Paleozoic era. Judging by its antiquity, there should not be any brown rock in the pool. However, something slowed down the decomposition of part of the layers.
The Pechersk coal basin is also located in western Russia. Its northern location makes mining difficult. In addition, it is located at a depth of hundreds of meters. We have to dig mines. Therefore, energy types of coal are extracted from the depths. Brown deposits are avoided.
Promising coal deposits in the north also include Taimyrskoye. From the name it is clear that the lakes are located on the sea border of the Krasnoyarsk Territory.

Brown coal deposit
Currently, geological exploration is underway in this area. Mining is being delayed. We'll have to resort to the mines again. So far, open reserves of the rock have not been depleted.
Of the total number of coal deposits in the world, about 50 are actively being developed. Many deposits remain in reserve and in. By the way, it is among the leaders in coal production, but not in first place. The USA occupied it. Coal-producing states there include Texas, Pennsylvania, Alabama, Colorado and Illinois.
It ranks 2nd in the world in coal mining, which includes brown rock. Usually, they cite the top ten, with Mongolia at the bottom. But let us also indicate . It went to the PRC. The Shanxing pool is being developed there. It occupies almost the entire Great Chinese Plain, extending into the Yangtze and Datong.
Application of brown coal
The use of brown coal depends on its type. Geologists distinguish 5. The first is “Dense”. It is the most valuable, bordering on stone. It is a dark, homogeneous, compacted rock.
It contains the maximum amount of hydrocarbons for brown coal. Like the stone version, the “Dense” fossil is shiny, but not pronounced. Not only private owners, but also small boiler houses are ready to use such fuel.
The second type of brown coal is “Earthy”. This breed is easily ground into powder. The raw material is suitable for semi-coking. This is the name for processing in a vacuum at a temperature of about 500 degrees Celsius. The result is charcoal. It burns well, does not produce smoke, and therefore is used both in everyday life and in industry.
Third type of brown coal- “Resinous.” It is dense and dark. Instead of an anthracite sheen, there is a resinous sheen. Such rock is distilled into liquid hydrocarbon fuel and, like peat coal.
The latter is slightly different from the usual one. Coal is, in fact, a relative with it. Both substances are products of the decomposition of plant organic matter. It is believed that peat is the first stage, and coals, starting with brown, are the subsequent ones.
It remains to mention the 5th type of brown coal – “Paper”. It is also called “Dizodil”. The rock is decayed plant matter. The layers are still clearly visible in it.

The photo shows brown coal burning
“Dizodil” can be made out by them, as if. Such coal, as a rule, is not used. The remaining types are fuel in one form or another. High-quality gasoline, for example, from the hero of the article is obtained by hydrogenation.
Begins brown coal processing from mixing rock with heavy oils. In the presence of a catalyst, the mixture is combined with. This requires heating up to 450 degrees Celsius. The output is not only liquid fuel, but also. It is a synthetic analogue of the natural one.
Finally, let us note the relationship between coal and humus. Who knows what will happen to the compost heap, leave it closed for millions of years... In general, there is a lot of .
They are beneficial to plants, causing rapid growth and fruiting. Therefore, some types of the hero of the article are used in fertilizers. As a rule, coal is mixed with vermicompost.
The proportions are the same. A prerequisite is grinding of brown rock. The coal fraction should not exceed 5 millimeters. Particles of 0.001 millimeter are preferred.
Brown coal price
On an industrial scale brown coal price stays within 900 - 1,400 per ton. For comparison, for 1,000 kilograms of coal in bulk purchases they ask for at least 1,800 rubles.
Usually, the price tag is about 2,500. A maximum of 4,000 rubles per ton is asked for anthracite. However, as with any website, there are exorbitant and very modest offers.
For example, brown coal can be sold in kilograms for 350 rubles. The offer is intended for gardeners. When preparing seedlings for the summer season, they do not see the difference with the price tags for fertilizers from stores; on the contrary, they see the benefits.
In part, the price tag for brown coal, like others, depends on the fraction. Large “cobblestones” are cheaper. Coal dust is inconvenient to handle, and therefore is also available. The most valued breed is the middle fraction.
As already mentioned, it also affects the name of the field. Industrialists know where to expect high-quality goods and where to get second-rate goods, and take into account the nuances of the rock composition in different deposits.

Transportation of brown coal
It was also mentioned that the method of coal mining is involved in pricing. Maintaining mines is expensive. By the way, the first coal mine was established in Holland. The date is surprising - 113th year.
So, the coal industry flourished in the Middle Ages. Moreover, the hero of the article and his “brothers” are recognized as the first type of fossil fuel that people began to use.
According to scientists, there are another 500 years ahead. There will not be enough proven coal reserves for a longer period. So, it is not surprising that there are active attempts to find alternative fuel sources to hydrocarbons.
Plants do not have time to rot at the rate at which humanity uses the hero of the article. In addition, in recent geological eras, the planet’s climate has changed, and coal formation has slowed down sharply.
All people have known the rich potential of charcoal since time immemorial. There are a lot of legends and stories associated with charcoal. About 6,000 years ago, charcoal was the main fuel for smelting copper. It was in great demand all over the world. Charcoal became very popular thanks to America's vast forests. Famous people such as Henry Ford, Stafford Orin made enormous contributions to charcoal production methods. The unique properties of charcoal allow it to be used in cooking. Charcoal has found fairly widespread use for this purpose in Japan.
What is charcoal really like? - you ask. Some consider it “nasty stuff.” Charcoal has long been known for all its beneficial properties and qualities. What interesting things do you know about charcoal?
Charcoal is one of the most suitable fuels for use. It produces virtually no smoke or open flame if ignited correctly. Charcoal only produces heat. Charcoal is an insulating material during construction; charcoal is also very hygroscopic and absorbs odors well. Charcoal is particularly well used in grilling and barbecuing. In cooking, briquettes are most often used - combined with other materials and formed into homogeneous elements. Quite often they are used in the cooking of American countries. According to the Barbecue Industries Association, Americans purchased 883,748 tons of charcoal briquettes in 1997.
Charcoal production relies on burning carbon-rich material, such as wood, in a low-oxygen atmosphere. This process removes moisture and volatile gases that are present in the wood. The resulting charred material not only burns longer and more consistently than wood, but also weighs much less.
Charcoal has been known since prehistoric times. About 5,300 years ago, an unfortunate traveler died in Tyrolean, in the Alps. Recently, when his body was found in a glacier, scientists saw that he was carrying a small box containing pieces of charred wood wrapped in maple leaves. The man did not have any tools for starting fire, such as flint, etc., so he may have been carrying smoldering charcoal.
About 6,000 years ago, charcoal was the main fuel for smelting copper. Following the invention of the blast furnace around 1400 AD, charcoal was used extensively throughout Europe for smelting metals. By the 18th century, forestry was depleted. I had to switch to an alternative fuel - coke.
The vast forests of eastern North America made charcoal widely used, especially in blacksmithing. By the late 19th century, it was also used in the western United States to extract silver from ore, as steam locomotive fuel and for heating residential and commercial buildings.
Around 1920, when Henry Ford (the owner of a car manufacturing plant) proposed pressing charcoal into briquettes, it began to be used not only as an industrial fuel, but also in cooking. Henry Ford began to profitably use sawdust and charcoal lumber produced at his automobile factory, and he also began to encourage the use of his own cars for going on picnics. Ford barbecue grills and charcoal were sold at the company's automobile dealerships, some of which devoted half the space to selling culinary products.
According to historical facts, charcoal was made by folding wood into a cone shape and covering it with dirt, peat and ash, leaving only a hole at the top to allow air to escape. The wood was distributed so that it burned slowly, and the air holes were distributed so that the resulting product cooled slowly. Modern charcoal pits were structures made of stone, brick, or a kiln that held 25 to 75 cords of wood (1 cord = 4 ft x 4 ft x 8 ft). A huge amount of forest could burn for 3 - 4 weeks and cool down in 7 - 10 days. This is a method of producing charcoal that produces a significant amount of smoke. In fact, changes in the color of the smoke signal transitions to different stages of the process. Initially, its whitish hue indicates the presence of steam as water vapor is released from the wood. When other components of wood (for example, resin) are released, the smoke becomes yellowish. Finally, the smoke turns bluish, indicating that charring has occurred completely. This is a good time to turn off the fire and cool the stove.
An alternative method of producing charcoal was developed in the early 1900s by Stafford Orin. It was he who helped Henry Ford develop his briquette business. Plants based on this method pass wood through a series of hearths or ovens. This is a continuous process. It consists in the fact that one end of the log is in the oven, and the other end is charred. In the traditional process, the wood is burned in a kiln and then grouped into groups. In fact, no visible smoke is released into the atmosphere because the gas emission methods can be effectively controlled.
Over the past few decades, charcoal and its production methods have changed quite a bit. The most significant innovation in recent years has been the development of easily recyclable briquettes. The unique thing is that charcoal briquettes are cooked within 10 minutes.
Unique facts about charcoal
- A mummy was found during excavations in China. As it was established, this was a 53-year-old woman who died of heart disease. This mummy is 2100 years old, but it looked like a 4-day-old corpse. There were more than 170 melon seeds in her belly. An experiment was conducted on these seeds, which showed that they all sprouted. These facts were soon explained by the fact that the diggers found 5 tons of charcoal at the base of the grave. It seems that all once living things were preserved for 2,000 years thanks to billions of negative ions made from charcoal!
- A huge number of Japanese companies use charcoal in the construction of foundations, factories, offices, and houses. Statistics show that people who work and live in buildings built with charcoal are less tired. The use of charcoal in building construction results in less destruction and longer machine life.
- The Japanese often use charcoal in cooking: it is added to oil for frying, so it does not taste bitter and can be used for several days while the charcoal is preserved in the oil.
- Studies have shown that adding charcoal powder to food has a positive effect on human health. 1 gram per day is considered sufficient. Charcoal powder is often added to buckwheat flour from which noodles are made. The effect of this addition is an increase in beneficial bacteria and a decrease in harmful bacteria in the intestines. This minimizes the tendency of the human body to lose beneficial bacteria until old age. Many health experts believe that the development of diseases such as cancer and premature aging depend on the development of microflora in the intestines. The more beneficial bacteria present in the body, the more effective the immune system becomes, making the body less prone to illness.
Publication of Pelleta.Com.Ua materials is permitted only with a link to Pelleta.Com.Ua. For news and online publications, a direct hyperlink, open to search engines, in the first paragraph to the cited article or news is mandatory.
The message about coal can be used in preparation for the lesson. The story about coal for children can be supplemented with interesting facts.
Report on coal
Coal is a solid, exhaustible, non-renewable mineral that humans use to produce heat by burning it. According to the classification, it belongs to sedimentary rocks. People began to use coal as an energy source in ancient times, along with firewood.
How is coal formed?
Coal appeared on Earth about 300-350 million years ago, when tree ferns grew luxuriantly in primeval swamps and the first gymnosperms began to appear.
Coal is believed to have formed from wood deposition. There were ancient forests, the trees of which accumulated in swamps, where, without access to oxygen, the activity of bacteria decomposing plant debris is reduced to zero, peat is formed, and then, in the process of burying these residues, coal is formed under high pressure and temperature.
Thus, for the formation of coal, peat must lie at a depth of three kilometers. At this depth, a layer of peat twenty meters will turn into coal with a layer thickness of two meters.
Types of coal
All types of coal occur in layers and their locations are called coal basins. Today, different types of coal are mined.
- Anthracites are the hardest varieties from great depths and have a maximum combustion temperature.
- Hard coal - many varieties mined in mines and in open pits. It is widely used in many areas of human activity.
- Brown coal - formed from the remains of peat, the youngest type of coal. Has the lowest combustion temperature.
How is coal mined?
Previously, coal was simply collected in places where the seam came to the surface. This could have happened as a result of displacement of layers of the earth's crust.
Often, after landslides in mountainous areas, such deposits were exposed, and people were able to get to pieces of “combustible stone.”
Later, when the first technology appeared, coal began to be mined using the open pit method. Some coal mines sank to depths of more than 300 meters.
Today, thanks to modern technology, people descend to a depth of more than 1000 m, where high-quality coal is mined.
Different types of coal can be used to produce heat. When burned, it is released in much greater quantities than can be obtained from firewood or other solid fuels. The hottest types of coal are used in metallurgy, where high temperatures are required.
In addition, coal is a valuable raw material for the chemical industry. Many necessary and useful substances are extracted from it.
We hope the information provided about coal helped you. You can leave your report about coal using the comment form.
Coal is a sedimentary rock that forms in the earth's formation. Coal is an excellent fuel. It is believed that this is the most ancient type of fuel that our distant ancestors used.
How is coal formed?
To form coal, a huge amount of plant matter is required. And it is better if the plants accumulate in one place and do not have time to decompose completely. The ideal place for this is swamps. The water in them is poor in oxygen, which prevents the life of bacteria.
Plant matter accumulates in swamps. Without having time to completely rot, it is compressed by subsequent soil deposits. This is how peat is obtained - the source material for coal. The following layers of soil seem to seal the peat in the ground. As a result, it is completely deprived of oxygen and water and turns into a coal seam. This process is long. Thus, most of the modern reserves of coal were formed in the Paleozoic era, i.e. more than 300 million years ago.
Characteristics and types of coal
(Brown coal)
The chemical composition of coal depends on its age.
The youngest species is brown coal. It lies at a depth of about 1 km. There is still a lot of water in it - about 43%. Contains a large amount of volatile substances. It ignites and burns well, but produces little heat.
Hard coal is a sort of “middle peasant” in this classification. It lies at depths of up to 3 km. Since the pressure of the upper layers is greater, the water content in coal is less - about 12%, volatile substances - up to 32%, but carbon contains from 75% to 95%. It is also flammable, but burns better. And due to the small amount of moisture it gives more heat.
Anthracite- an older breed. It lies at depths of about 5 km. It contains more carbon and virtually no moisture. Anthracite is a solid fuel and does not ignite well, but the specific heat of combustion is the highest - up to 7400 kcal/kg.
(Anthracite coal)
However, anthracite is not the final stage of transformation of organic matter. When exposed to more severe conditions, coal transforms into shuntite. At higher temperatures, graphite is obtained. And under ultra-high pressure, coal turns into diamond. All these substances - from plants to diamonds - are made of carbon, only the molecular structure is different.
In addition to the main “ingredients,” coal often includes various “rocks.” These are impurities that do not burn, but form slag. Coal also contains sulfur, and its content is determined by the place where coal is formed. When burned, it reacts with oxygen and forms sulfuric acid. The less impurities in the composition of coal, the higher its grade is valued.
Coal deposit

The location of hard coal is called a coal basin. There are over 3.6 thousand coal basins known in the world. Their area occupies about 15% of the earth's land area. The largest percentage of the world's coal reserves is in the United States - 23%. In second place is Russia, 13%. China closes the top three countries with 11%. The largest coal deposits in the world are located in the USA. This is the Appalachian coal basin, whose reserves exceed 1,600 billion tons.
In Russia, the largest coal basin is Kuznetsk, in the Kemerovo region. Kuzbass reserves amount to 640 billion tons.
The development of deposits in Yakutia (Elginskoye) and Tyva (Elegestskoye) is promising.
Coal mining

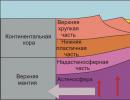
Depending on the depth of coal occurrence, either closed or open mining methods are used.
Closed or underground mining method. For this method, mine shafts and adits are built. Mine shafts are built if the depth of coal is 45 meters or higher. A horizontal tunnel leads from it - an adit.
There are 2 closed mining systems: room and pillar mining and longwall mining. The first system is less economical. It is used only in cases where the discovered layers are thick. The second system is much safer and more practical. It allows you to extract up to 80% of the rock and evenly deliver coal to the surface.
The open method is used when the coal lies shallow. To begin with, they analyze the hardness of the soil, determine the degree of weathering of the soil and the layering of the covering layer. If the soil above the coal seams is soft, the use of bulldozers and scrapers is sufficient. If the upper layer is thick, then excavators and draglines are brought in. The thick layer of hard rock lying above the coal is blasted.
Application of coal

The area of use of coal is simply enormous.
Sulfur, vanadium, germanium, zinc, and lead are extracted from coal.
Coal itself is an excellent fuel.
Used in metallurgy for iron smelting, in the production of cast iron and steel.
The ash obtained after burning coal is used in the production of building materials.
From coal, after special processing, benzene and xylene are obtained, which are used in the production of varnishes, paints, solvents, and linoleum.
By liquefying coal, first-class liquid fuel is obtained.
Coal is the raw material for the production of graphite. As well as naphthalene and a number of other aromatic compounds.
As a result of chemical processing of coal, over 400 types of industrial products are currently obtained.
This article provides information about an interesting sedimentary rock that is a source of great economic importance. This rock, amazing in the history of its origin, is called “coal”. His education is quite interesting. It should be noted that, despite the fact that this rock makes up less than one percent of all sedimentary rocks existing on earth, it is of great importance in many areas of human life.
general information
How was coal formed? Its formation includes many processes occurring in nature.
Coal appeared on Earth approximately 350 million years ago. To explain it in a simple way, it happened as follows. Tree trunks, falling into the water with other vegetation, gradually formed huge layers of organic, undecomposed mass. The limited access of oxygen did not allow this mess to decompose and rot, which gradually sank deeper and deeper under its weight. Over a long period of time and due to the displacement of layers of the earth's crust, these layers went to a considerable depth, where, under the influence of elevated temperatures and high pressure, this mass was converted into coal.
Below we will take a closer look at how coal appeared, the formation of which is very interesting and curious.

Types of coal
In modern coal deposits around the world, different types of coal are mined:
1. Anthracite. These are the hardest varieties, mined from great depths and having the highest combustion temperature.
2. Coal. Many of its varieties are mined in open pits and mines. This type is the most common in areas of human activity.
3. Brown coal. This is the youngest species, formed from peat residues and has the lowest combustion temperature.
All of the listed forms of coal lie in layers, and the places where they accumulate are called coal basins.
Theories of the origin of coal
What is coal? Simply put, this sediment is accumulated, compacted and processed plants over time.
There are two theories, the more popular of which is the one that many geologists adhere to. It is as follows: the plants that make up coal accumulated in large peat or freshwater swamps for many thousands of years. This theory assumes the growth of vegetation in the place where the rocks were discovered and is called “autochthonous”.

Another theory is based on the fact that coal seams accumulated from plants transported from other places, which were deposited in a new area under flood conditions. In other words, coal originated from transported plant debris. The second theory is called allochthonous.
In both cases, the source of coal formation is plants.
Why is this stone burning?
The main chemical element in coal that has beneficial properties is carbon.
Depending on the conditions of formation, processes and age of the layers, each coal deposit contains its own certain percentage of carbon. This indicator determines the quality of natural fuel, since the level of heat transfer is directly related to the amount of carbon oxidized during the combustion process. The higher the calorific value of a given rock, the more suitable it is as a source of heat and energy.
What is coal for people around the world? First of all, this is the best fuel, suitable for various spheres of life.

About fossils in coal
The fossil plant species found in coal do not support the autochthonous theory of origin. Why? For example, moss trees and giant ferns, characteristic of the coal deposits of Pennsylvania, could grow in swampy conditions, while other fossil plants of the same basin (conifers or giant horsetail, etc.) preferred drier soils rather than swampy places. It turns out that they were somehow transported to these places.
How did coal come into being? The formation in nature is amazing. Marine fossils such as molluscs, fish and brachiopods (or brachiopods) are also common in coal. In coal seams there are also coal balls (rounded, crumpled masses of perfectly preserved fossil plants and animals, including marine ones). For example, the small annelid sea worm is commonly found attached to plants in coals of North America and Europe. They belong to the Carboniferous period.
The occurrence of marine animals interspersed with non-marine plants in coal sedimentary rocks indicates that they mixed during the movement. Amazing and lengthy processes took place in nature before coal was finally formed. Its formation in this way confirms the allochthonous theory.
Amazing Finds
The most interesting finds in the coal layers are tree trunks lying vertically. They often cross huge strata of rock perpendicular to the coal bedding. Trees in this vertical position are often found in layers associated with coal deposits, and a little less often in the coal itself. Many are of the opinion about moving tree trunks.

The amazing thing is that sediment had to accumulate so quickly to cover these trees before they deteriorated (rotted) and fell.
Here is a rather interesting history of the formation of a rock called coal. The formation of such layers in the bowels of the earth is a reason for further research in search of answers to numerous questions.
Where do the lumps in coal come from?
An impressive external feature of coal is that it contains huge lumps. These large blocks have been found in coal seams of many deposits for more than a hundred years. The average weight of 40 lumps collected from the West Virginia coalfield was about 12 pounds, and the largest was 161 pounds. Moreover, many of them were metamorphic or volcanic rock.

Researcher Price suggested that they could have been transported to the coal deposits in Virginia from afar, entwined in the roots of trees. This conclusion also supports the allochthonous model of coal formation.
Conclusion
Many studies prove the truth of the allochthonous theory of coal formation: the presence of remains of terrestrial and marine animals and plants implies their movement.
Studies have also proven that the metamorphism of this rock does not require a long time (millions of years) of exposure to pressure and heat - it can also form as a result of rapid heating. And trees located vertically in coal sediments confirm the rather rapid accumulation of vegetation remains.
Activated (active) carbon is a porous substance that is obtained from various carbon-containing materials of organic origin: charcoal (grades of activated carbon BAU-A, OU-A, DAK, etc.), coal coke (grades of activated carbon AG-3, AG- 5, AR, etc.), petroleum coke, coconut charcoal, etc. It contains a huge number of pores and therefore has a very large specific surface area per unit mass, as a result of which it has high adsorption. 1 gram of activated carbon, depending on the manufacturing technology, has a surface area from 500 to 1500 m2. Used in medicine and industry for purification, separation and extraction of various substances.

Activated carbon

How coal works:

Activated carbon

There are two main mechanisms by which activated carbon removes contaminants from water: adsorption and catalytic reduction (a process that causes negatively charged contaminant ions to be attracted to positively charged activated carbon). Organic compounds are removed by adsorption, and residual disinfectants such as chlorine and chloramines are removed by catalytic reduction.
Production:
Good activated carbon is obtained from nut shells (coconut shells, from the seeds of some fruit crops.) Previously, activated carbon was made from cattle bones (bone charcoal). The essence of the activation process is the opening of pores that are in a closed state in the carbon material. This is done either thermochemically (the material is first impregnated with a solution of zinc chloride, potassium carbonate or some other compounds and heated without air access), or by treatment with superheated steam or carbon dioxide or a mixture thereof at a temperature of 800-850 degrees. In the latter case, it is technically difficult to obtain a vapor-gas agent having such a temperature. A widespread technique is to supply a limited amount of air into the apparatus for activation simultaneously with saturated steam. Part of the coal burns and the required temperature is reached in the reaction space. The yield of active carbon in this process variant is noticeably reduced. The specific pore surface area of the best brands of active carbons can reach 1800-2200 m2; per 1 g of coal. There are macro-, meso- and micropores. Depending on the size of the molecules that need to be retained on the surface of the coal, coal must be produced with different pore size ratios.
Application:
1) Wearing gas masks
A classic example of the use of activated carbon is associated with its use in a gas mask. The gas mask developed by N.D. Zelinsky saved many lives of soldiers in the First World War. By 1916 it had been adopted by almost all European armies;
2)In the production of sugar
Initially, bone activated carbon was used to clean sugar syrup from coloring substances during sugar production. However, this sugar could not be consumed during fasting, as it was of animal origin. Sugar refineries began producing “fast sugar,” which was either unrefined and looked like colored fondant, or refined through charcoal;
3)Other applications
Activated carbon is used in medicine, chemicals, as a carrier of catalysts, and in many reactions it itself acts as a catalyst, in the pharmaceutical and food industries. Filters containing activated carbon are used in many modern models of drinking water purification devices.
This included both food preparation and industrial production. Coal made it possible to make steel. There are many interesting facts associated with coal, and its role in our lives is colossal.
The formation of coal in the bowels of the earth is a very long process. This has a lot in common with oil. Coal is formed from dead plants that, for one reason or another, ended up underground. Here, without oxygen, they did not rot, and their remains did not lose the carbon they contained - the basis of coal. Then, over the course of millions of years, under the influence of a variety of factors, these remains turned into peat, and from it into coal. And the further process leads to the formation of graphite.
Before delving into interesting facts about mining technology and interesting situations related to coal, let's talk about the coals needed for cooking:
In general, the main difference between Japanese cuisine and European cuisine is the dominance of seafood. They are used everywhere. And even for kebabs, which the Japanese call “tempora”. True, they do not very often use coal for their preparation. It is believed that it is able to absorb odors and then release them to the prepared dish. Open fires are generally preferred to coal. In addition, ginger is often used, which also eliminates odors.
Coal mines are quite dangerous places. They release various gases. Methane is especially dangerous. It displaces some of the oxygen and makes the air explosive. In the past, when methane indicators did not exist, canaries were used. They were taken into the mine with them, and if the birds became ill, this meant that methane had accumulated in the mine.
In North Africa, French-speaking countries like to use dry bushes and other small plants. There is a desert here and there are no large trees. Coals are made, for example, from saxaul. They turn out hot and have a specific aroma.
Among other dangers, fires in mines stand out. As in the case of burning peat, they can last quite a long time. A record-breaking fire occurred at the Liuhuangou oil field in China. It took 130 years to eliminate it, and it was finally extinguished only in 2004. About 260 million tons of coal were destroyed.
There are many funny situations associated with coal and its deposits. Treasures were often found in it. So in 1891, a certain Mrs. Culp was lucky when she found an old gold chain in a large piece of coal. Coal holds many ancient artifacts. Miners have repeatedly found the remains of ancient structures. As, for example, in the American town of Hammondville, where in 1869 the remains of a wall with hieroglyphs were found.
Coal continues to play a big role in the lives of people and even entire cities. It is interesting to trace the fate of the Japanese city of Hashima, located on the island of the same name, which was once rich in coal. Since the 1930s, this city has long been considered the most populous in the world. The island had a coastline of only 1 km, but its population was more than 5 thousand people. But by the mid-70s, coal ran out here. People began to leave this place. The city became completely abandoned. Now they even conduct extreme excursions there.
Coal can have more than just the usual solid form. Today there are technologies that turn it into liquid fuel, very similar to oil.
In industry, coal is used not only as fuel. It is a raw material for the production of various materials. For example, artificial graphite is made from coal. The useful materials it contains are also extracted from coal: lead, sulfur, gallium, zinc and others.
All people have known the rich potential of charcoal since time immemorial. There are a lot of legends and stories associated with charcoal. About 6,000 years ago, charcoal was the main fuel for smelting copper. It was in great demand all over the world. Charcoal became very popular thanks to America's vast forests. Famous people such as Henry Ford, Stafford Orin made enormous contributions to charcoal production methods. The unique properties of charcoal allow it to be used in cooking. Charcoal has found fairly widespread use for this purpose in Japan.
What is charcoal really like? - you ask. Some consider it “nasty stuff.” Charcoal has long been known for all its beneficial properties and qualities. What interesting things do you know about charcoal?
Charcoal is one of the most suitable fuels for use. It produces virtually no smoke or open flame if ignited correctly. Charcoal only produces heat. Charcoal is an insulating material during construction; charcoal is also very hygroscopic and absorbs odors well. Charcoal is particularly well used in grilling and barbecuing. In cooking, briquettes are most often used - combined with other materials and formed into homogeneous elements. Quite often they are used in the cooking of American countries. According to the Barbecue Industries Association, Americans purchased 883,748 tons of charcoal briquettes in 1997.
Charcoal production relies on burning carbon-rich material, such as wood, in a low-oxygen atmosphere. This process removes moisture and volatile gases that are present in the wood. The resulting charred material not only burns longer and more consistently than wood, but also weighs much less.
Charcoal has been known since prehistoric times. About 5,300 years ago, an unfortunate traveler died in Tyrolean, in the Alps. Recently, when his body was found in a glacier, scientists saw that he was carrying a small box containing pieces of charred wood wrapped in maple leaves. The man did not have any tools for starting fire, such as flint, etc., so he may have been carrying smoldering charcoal.
About 6,000 years ago, charcoal was the main fuel for smelting copper. Following the invention of the blast furnace around 1400 AD, charcoal was used extensively throughout Europe for smelting metals. By the 18th century, forestry was depleted. I had to switch to an alternative fuel - coke.
The vast forests of eastern North America made charcoal widely used, especially in blacksmithing. By the late 19th century, it was also used in the western United States to extract silver from ore, as steam locomotive fuel and for heating residential and commercial buildings.
Around 1920, when Henry Ford (the owner of a car manufacturing plant) proposed pressing charcoal into briquettes, it began to be used not only as an industrial fuel, but also in cooking. Henry Ford began to profitably use sawdust and charcoal lumber produced at his automobile factory, and he also began to encourage the use of his own cars for going on picnics. Ford barbecue grills and charcoal were sold at the company's automobile dealerships, some of which devoted half the space to selling culinary products.
According to historical facts, charcoal was made by folding wood into a cone shape and covering it with dirt, peat and ash, leaving only a hole at the top to allow air to escape. The wood was distributed so that it burned slowly, and the air holes were distributed so that the resulting product cooled slowly. Modern charcoal pits were structures made of stone, brick, or a kiln that held 25 to 75 cords of wood (1 cord = 4 ft x 4 ft x 8 ft). A huge amount of forest could burn for 3 - 4 weeks and cool down in 7 - 10 days. This is a method of producing charcoal that produces a significant amount of smoke. In fact, changes in the color of the smoke signal transitions to different stages of the process. Initially, its whitish hue indicates the presence of steam as water vapor is released from the wood. When other components of wood (for example, resin) are released, the smoke becomes yellowish. Finally, the smoke turns bluish, indicating that charring has occurred completely. This is a good time to turn off the fire and cool the stove.
An alternative method of producing charcoal was developed in the early 1900s by Stafford Orin. It was he who helped Henry Ford develop his briquette business. Plants based on this method pass wood through a series of hearths or ovens. This is a continuous process. It consists in the fact that one end of the log is in the oven, and the other end is charred. In the traditional process, the wood is burned in a kiln and then grouped into groups. In fact, no visible smoke is released into the atmosphere because the gas emission methods can be effectively controlled.
Over the past few decades, charcoal and its production methods have changed quite a bit. The most significant innovation in recent years has been the development of easily recyclable briquettes. The unique thing is that charcoal briquettes are cooked within 10 minutes.
Unique facts about charcoal
- A mummy was found during excavations in China. As it was established, this was a 53-year-old woman who died of heart disease. This mummy is 2100 years old, but it looked like a 4-day-old corpse. There were more than 170 melon seeds in her belly. An experiment was conducted on these seeds, which showed that they all sprouted. These facts were soon explained by the fact that the diggers found 5 tons of charcoal at the base of the grave. It seems that all once living things were preserved for 2,000 years thanks to billions of negative ions made from charcoal!
- A huge number of Japanese companies use charcoal in the construction of foundations, factories, offices, and houses. Statistics show that people who work and live in buildings built with charcoal are less tired. The use of charcoal in building construction results in less destruction and longer machine life.
- The Japanese often use charcoal in cooking: it is added to oil for frying, so it does not taste bitter and can be used for several days while the charcoal is preserved in the oil.