Biological significance of oxygen. Biological role. Being in nature
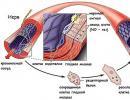
Oxygen is part of all vital organic substances - proteins, fats, carbohydrates. Without oxygen, the processes of respiration, oxidation of amino acids, fats, and carbohydrates are impossible. In higher animals, oxygen enters the blood, combining with hemoglobin to form oxyhemoglobin. Oxyhemoglobin HbO 2 in the capillaries gives up oxygen HbO 2 ® Hb + O 2 through the walls of the capillaries. O 2 (oxygen) enters the cells, where it is spent on the oxidation of various substances, as a result of these processes CO 2 and H 2 O are formed, energy is released:
Hb + CO 2 ® HbCO 2 (carboxyhemoglobin)
The allotropic modification of oxygen, ozone – O 3, plays a certain role in the formation of radicals. These radicals initiate radical chain reactions with bioorganic molecules - lipids, proteins, DNA, which leads to cell death. This is the basis for the effect of ozone on microorganisms contained in air and water. Therefore, O 3 is used for ozonation of air, disinfection of drinking water, and swimming pool water. In an atmosphere with excess ozone content (its source is exhaust gases), radicals (RO 2 ·; OH ·) are formed in the human body, which can initiate tumor diseases. In addition, ozone plays an important role in protecting biological objects of the Earth from hard X-ray radiation, because At an altitude of ~25 km, an ozone layer is formed that absorbs rays with l £ 260 nm.
Of the oxygen compounds, H 2 O 2 and H 2 O are very important. The human body contains about 80% water. Due to its structure (two sp 3 hybrid orbitals are connected, two contain a lone pair of electrons), water has a very high dipole moment and is therefore a universal solvent. In the human and animal body, it dissolves organic and inorganic substances and promotes their ionization (dissociation). Water is both a medium in which biochemical reactions take place and a participant in the reactions of hydrolysis of fats, ATP, ADP, etc.
Biological role of hydrogen peroxide
In mitochondria, H atoms cleaved from the substrate in the form of H + under the action of dehydroginase bind to oxygen, forming water.
4H + + O 2 + 4e - ® 2H 2 O
In this case, it is important to add exactly 4 electrons, because when 2 electrons are added, hydrogen peroxide is formed
2H + + O 2 + 2e - ® H 2 O 2
When one electron is added, a hyperoxide ion is formed
O 2 · + e - ® O 2 -
Hydrogen peroxide and hyperoxide radical O 2 are toxic to cells because they interact with the lipids of cell membranes and disable them, disrupt the structure of the cell, including DNA and its reparative function. Aerobic cells, using the enzyme catalase and superoxide dismutase (copper-containing enzyme), convert H 2 O 2 and O 2 - into O 2
2O 2 - + 2H + 2O - + 2H + H 2 O 2 + O 2
2H 2 O 2 2H 2 O + O 2
Application in medicine. Medications
Oxygenium(O 2) – oxygen. It is introduced into the body by inhalation in case of cardiovascular failure, relieves oxygen starvation (hypoxia). It is introduced into the gastrointestinal tract through a probe for helminthiasis (roundworms, whipworms).
Aqua purificata(H 2 O) – purified water. Used for the preparation of liquid dosage forms.
Solutiohydrogenii peroxydidiluta(3%) – hydrogen peroxide solution (3%).
Perhydrolum (33-35%) perhydrol. Hydrogen peroxide solution 33-35% .
Magnesii peroxydum,(MgO 2 ´MgO) – magnesium peroxide.
Hydroperitum(H 2 O 2 ´NH 2 -CO-NH 2) – hydroperite (contains 0.08% citric acid).
Hydrogen peroxide preparations are used externally to treat wounds, rinse the mouth and throat as an antiseptic and deodorizing agent.
Sulfur
Sulfur is an element of the main subgroup of group VI of the periodic table
DI. Mendeleev. In this group, starting from sulfur (3rd period), a d-sublevel appears, therefore the number of unpaired electrons can increase from 2 to 4 and 6, due to the pairing of s- and p-electrons and moving them to the d-sublevel:
Thus, the possible and manifested oxidation states of sulfur are: -2, +2, +4 and +6.
From top to bottom in the subgroup from oxygen to polonium, the atomic sizes increase, and the ionization energy decreases, and the nonmetallic properties in the series: O – S – Se – Te – Po weaken.
Sulfur is a typical non-metal; in terms of its OEO value (2.5), it is second only to halogens, oxygen and nitrogen.
Sulfur is one of the common elements. In the earth's crust its content is 0.05 wt. %, in sea water 0.08 - 0.09%. It consists of four stable isotopes: 32 S (95.084%), 33 S (0.74%), 34 S (4.16%), and 36 S (0.016%). Radioactive isotopes of sulfur were obtained: 31 S (T 1/2 = 2.66 sec.), 35 S (T 1/2 = 86.3 days) and 37 S (T 1/2 = 5.07 min.).
Sulfur occurs in nature in a native state (mostly near volcanoes and in hot mineral springs, as a product of the oxidation of hydrogen sulfide).
It was used to prepare paints, as a medicinal product, and also for other purposes.
Sulfur is found in various rocks: limestone, calcite, gypsum, etc.; in sulfur ores and minerals, in living and plant organisms (0.16% in the human body, is a macroelement), i.e. in many inorganic and organic compounds. Main sulfur minerals:
Oxygen is an organogenic element. Its content makes up up to 65% of a person’s body weight, that is, more than 40 kg for an adult. Oxygen is the most common oxidizing agent on Earth; in the environment it is presented in two forms - in the form of compounds (the earth's crust and water: oxides, peroxides, hydroxides, etc.) and in free form (the atmosphere).
Biological role of oxygen
The main (in fact, the only) function of oxygen is its participation as an oxidizing agent in redox reactions in the body. Thanks to the presence of oxygen, the organisms of all animals are able to utilize (actually “burn”) various substances ( carbohydrates, fats, squirrels) with the extraction of a certain “combustion” energy for its own needs. At rest, the adult body consumes 1.8-2.4 g of oxygen per minute.
Oxygen sources
The main source of oxygen for humans is the Earth’s atmosphere, from where, through respiration, the human body is able to extract the amount of oxygen necessary for life.
Oxygen deficiency
When there is a deficiency in the human body, so-called hypoxia develops.
Causes of oxygen deficiency
- absence or sharply reduced oxygen content in the atmosphere;
- reduced partial pressure of oxygen in the inhaled air (when rising to high altitudes - in the mountains, in aircraft);
- cessation or reduction of oxygen supply to the lungs during asphyxia;
- disorders of oxygen transport (disorders of the cardiovascular system; a significant decrease in hemoglobin in the blood during anemia, the inability of hemoglobin to perform its functions - to bind, transport or release oxygen to tissues, for example, in case of carbon monoxide poisoning);
- inability of tissues to utilize oxygen due to disruption of redox processes in tissues (for example, cyanide poisoning)
Consequences of oxygen deficiency
In acute hypoxia:
- loss of consciousness;
- disorder, irreversible damage and rapid death of the central nervous system (literally in minutes)
- For chronic hypoxia:
- rapid physical and mental fatigue;
- disorders of the central nervous system;
- tachycardia and shortness of breath at rest or with little physical activity
Excess oxygen
It is observed extremely rarely, as a rule, in artificial conditions (for example, hyperbaric chambers, incorrectly selected mixtures for breathing when diving in water, etc.). In this case, prolonged inhalation of air excessively enriched with oxygen is accompanied by oxygen poisoning - as a result of its excessive amount, a large number of free radicals are formed in organs and tissues, and the process of spontaneous oxidation of organic substances, including lipid peroxidation, is initiated.
Daily requirement: not standardized
Plan:
History of discovery
Origin of name
Being in nature
Receipt
Physical properties
Chemical properties
Application
Biological role of oxygen
Toxic oxygen derivatives
10. Isotopes
Oxygen
Oxygen- element of the 16th group (according to the outdated classification - the main subgroup of group VI), the second period of the periodic system of chemical elements of D.I. Mendeleev, with atomic number 8. Denoted by the symbol O (lat. Oxygenium). Oxygen is a chemically active non-metal and is the lightest element from the group of chalcogens. Simple substance oxygen(CAS number: 7782-44-7) under normal conditions is a colorless, tasteless and odorless gas, the molecule of which consists of two oxygen atoms (formula O 2), and therefore it is also called dioxygen. Liquid oxygen has a light blue color, and solid crystals are light blue in color.
There are other allotropic forms of oxygen, for example, ozone (CAS number: 10028-15-6) - under normal conditions, a blue gas with a specific odor, the molecule of which consists of three oxygen atoms (formula O 3).
History of discovery
It is officially believed that oxygen was discovered by the English chemist Joseph Priestley on August 1, 1774 by decomposing mercuric oxide in a hermetically sealed vessel (Priestley directed sunlight at this compound using a powerful lens).
However, Priestley initially did not realize that he had discovered a new simple substance; he believed that he had isolated one of the constituent parts of air (and called this gas “dephlogisticated air”). Priestley reported his discovery to the outstanding French chemist Antoine Lavoisier. In 1775, A. Lavoisier established that oxygen is a component of air, acids and is found in many substances.
A few years earlier (in 1771), oxygen was obtained by the Swedish chemist Karl Scheele. He calcined saltpeter with sulfuric acid and then decomposed the resulting nitric oxide. Scheele called this gas “fire air” and described his discovery in a book published in 1777 (precisely because the book was published later than Priestley announced his discovery, the latter is considered the discoverer of oxygen). Scheele also reported his experience to Lavoisier.
An important step that contributed to the discovery of oxygen was the work of the French chemist Pierre Bayen, who published works on the oxidation of mercury and the subsequent decomposition of its oxide.
Finally, A. Lavoisier finally figured out the nature of the resulting gas, using information from Priestley and Scheele. His work was of enormous importance because thanks to it, the phlogiston theory, which was dominant at that time and hampered the development of chemistry, was overthrown. Lavoisier conducted experiments on the combustion of various substances and disproved the theory of phlogiston, publishing results on the weight of the burned elements. The weight of the ash exceeded the original weight of the element, which gave Lavoisier the right to claim that during combustion a chemical reaction (oxidation) of the substance occurs, and therefore the mass of the original substance increases, which refutes the theory of phlogiston.
Thus, the credit for the discovery of oxygen is actually shared between Priestley, Scheele and Lavoisier.
origin of name
The word oxygen (also called “acid solution” at the beginning of the 19th century) owes its appearance in the Russian language to some extent to M.V. Lomonosov, who introduced the word “acid”, along with other neologisms; Thus, the word “oxygen”, in turn, was a tracing of the term “oxygen” (French oxygène), proposed by A. Lavoisier (from ancient Greek ὀξύς - “sour” and γεννάω - “giving birth”), which is translated as “generating acid”, which is associated with its original meaning - “acid”, which previously meant substances called oxides according to modern international nomenclature.
Being in nature
Oxygen is the most common element on Earth; its share (in various compounds, mainly silicates) accounts for about 47.4% of the mass of the solid earth's crust. Sea and fresh waters contain a huge amount of bound oxygen - 88.8% (by mass), in the atmosphere the content of free oxygen is 20.95% by volume and 23.12% by mass. More than 1,500 compounds in the earth's crust contain oxygen.
Oxygen is part of many organic substances and is present in all living cells. In terms of the number of atoms in living cells, it is about 25%, and in terms of mass fraction - about 65%.
The discovery of oxygen happened twice, in the second half of the 18th century, several years apart. In 1771, oxygen was obtained by Swede Karl Scheele by heating saltpeter and sulfuric acid. The resulting gas was called "fire air". In 1774, the English chemist Joseph Priestley carried out the process of decomposing mercuric oxide in a completely closed vessel and discovered oxygen, but mistook it for an ingredient in air. Only after Priestley shared his discovery with the Frenchman Antoine Lavoisier did it become clear that a new element (calorizator) had been discovered. Priestley takes the lead in this discovery because Scheele published his scientific work describing the discovery only in 1777.
Oxygen is an element of group XVI of period II of the periodic table of chemical elements by D.I. Mendeleev, has atomic number 8 and atomic mass 15.9994. It is customary to denote oxygen by the symbol ABOUT(from Latin Oxygenium- generating acid). In Russian the name oxygen became a derivative of acids, a term that was introduced by M.V. Lomonosov.
Being in nature
Oxygen is the most common element found in the earth's crust and the World Ocean. Oxygen compounds (mainly silicates) make up at least 47% of the mass of the earth's crust; oxygen is produced during the process of photosynthesis by forests and all green plants, most of which comes from the phytoplankton of marine and fresh waters. Oxygen is an essential component of any living cells and is also found in most substances of organic origin.
Physical and chemical properties
Oxygen is a light non-metal, belongs to the group of chalcogens, and has high chemical activity. Oxygen, as a simple substance, is a colorless, odorless and tasteless gas; it has a liquid state - light blue transparent liquid and a solid state - light blue crystals. Consists of two oxygen atoms (denoted by the formula O₂).
Oxygen is involved in redox reactions. Living things breathe oxygen from the air. Oxygen is widely used in medicine. In case of cardiovascular diseases, to improve metabolic processes, oxygen foam (“oxygen cocktail”) is injected into the stomach. Subcutaneous administration of oxygen is used for trophic ulcers, elephantiasis, and gangrene. Artificial ozone enrichment is used to disinfect and deodorize air and purify drinking water.

Oxygen is the basis of the life activity of all living organisms on Earth and is the main biogenic element. It is found in the molecules of all the most important substances that are responsible for the structure and functions of cells (lipids, proteins, carbohydrates, nucleic acids). Every living organism contains much more oxygen than any element (up to 70%). For example, the body of an average adult human weighing 70 kg contains 43 kg of oxygen.
Oxygen enters living organisms (plants, animals and humans) through the respiratory system and the intake of water. Remembering that in the human body the most important respiratory organ is the skin, it becomes clear how much oxygen a person can receive, especially in the summer on the shore of a reservoir. Determining a person’s need for oxygen is quite difficult, because it depends on many factors - age, gender, body weight and surface area, nutrition system, external environment, etc.

Use of oxygen in life
Oxygen is used almost everywhere - from metallurgy to the production of rocket fuel and explosives used for road work in the mountains; from medicine to the food industry.
In the food industry, oxygen is registered as a food additive, as a propellant and packaging gas.
Oxygen is the most abundant element on Earth. Sea water contains 85.82% oxygen, atmospheric air contains 23.15% by weight or 20.93% by volume, and the earth's crust contains 47.2% by weight. This concentration of oxygen in the atmosphere is maintained constant by the process of photosynthesis. In this process, green plants convert carbon dioxide and water into carbohydrates and oxygen when exposed to sunlight. The bulk of oxygen is in a bound state; The amount of molecular oxygen in the atmosphere is only 0.01% of the total oxygen content in the earth's crust. In natural life, oxygen is of exceptional importance. Oxygen and its compounds are indispensable for maintaining life. They play a vital role in metabolic processes and respiration. Oxygen is part of proteins, fats, carbohydrates, from which organisms are “built”; The human body, for example, contains about 65% oxygen. Most organisms obtain the energy necessary to perform their vital functions through the oxidation of certain substances with the help of oxygen. The loss of oxygen in the atmosphere as a result of the processes of respiration, decay and combustion is compensated by oxygen released during photosynthesis. Deforestation, soil erosion, and various surface mining reduce the total mass of photosynthesis and reduce the cycle over large areas.
Oxygen was not always part of the earth's atmosphere. It appeared as a result of the vital activity of photosynthetic organisms. Under the influence of ultraviolet rays it turned into ozone. As ozone accumulated, an ozone layer formed in the upper atmosphere. The ozone layer, like a screen, reliably protects the Earth's surface from ultraviolet radiation, which is fatal to living organisms.
Geochemical oxygen cycle connects the gas and liquid shells with the earth's crust. Its main points: the release of free oxygen during photosynthesis, the oxidation of chemical elements, the entry of extremely oxidized compounds into the deep zones of the earth's crust and their partial reduction, including due to carbon compounds, the removal of carbon monoxide and water to the surface of the earth's crust and their involvement in the reaction photosynthesis.
In addition to the oxygen cycle described above in an unbound form, this element also completes the most important cycle, entering the composition of water (Fig. 3). During the cycle, water evaporates from the surface of the ocean, water vapor moves along with air currents, condenses, and water returns in the form of precipitation to the surface of land and sea. There is a large water cycle, in which water that falls as precipitation on land returns to the seas through surface and underground runoff; and the small water cycle, which deposits precipitation on the ocean surface.
The oxygen cycle is accompanied by its inflow and outflow.
The arrival of oxygen includes: 1) release during photosynthesis; 2) formation in the ozone layer under the influence of UV radiation (in small quantities); 3) dissociation of water molecules in the upper layers of the atmosphere under the influence of UV radiation; 4) formation of ozone – O3.
Oxygen consumption includes: 1) consumption by animals during breathing; 2) oxidative processes in the earth’s crust; 3) oxidation of carbon monoxide (CO) released during volcanic eruptions.